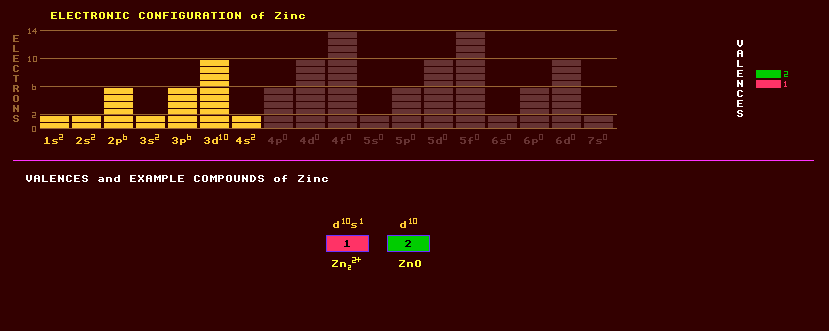

|
30 ZINC Zn (German, zink)
Zinc is a hard brittle white metal with a bluish tinge and is an essential trace element in humans, occurring in many foods. Deficiency in zinc leads first to anosmia, or loss of the sense of smell. It has a fairly low melting point of 418 Celsius and burns in air at red heat forming white clouds of the oxide, which if inhaled, can give rise to 'oxide shakes' or 'zinc chills'. Otherwise zinc and its compounds is not considered toxic. Zinc is alloyed with many metals, with copper it forms brass, diecast alloys contain mostly zinc with 3% Al, 0 - 3.5% Cu and a little Mg. A lightweight alloy called Prestel, 78% zinc and 22% aluminium, is as strong as steel but as mouldable as plastic. Other alloys are typewriter metal, nickel silver, German silver, spring brass and soft solder. A sacrificial zinc cathodic protector protects bronze propellers from corrosion. An alloy with zirconium, ZrZn2, is ferromagnetic below 35 Kelvin. Zinc finds extensive use to plate iron and steel, which is then known as galvanised iron, and protects it from corrosion. Zinc readily crystallizes into large crystals which on galvanised iron can be seen as large irregular shaped domains several centimetres across. Zinc oxide, ZnO, is a white powder used medicinally in zinc oxide plasters and ointments and as a non-poisonous pigment (zinc white) in paints. Zinc chromate (zinc yellow) is a yellow pigment used in zinc chrome paint. Zinc forms the outer cathode of ordinary dry cell batteries (zinc carbon Lechlanche cells). Higher power versions use zinc chloride as the electrolyte instead of ammonium chloride. Zinc telluride, a II/VI semiconductor, is used as a semiconductor capable of withstanding high temperatures (up to 750ºC). Zinc sulphide, ZnS, is used as a phosphor in CRTs, scintillation screens and fluorescent lights. Zinc sulphide, ZnS, can exist in two polymorphic forms having the same formula but different crystal structures; at low temperatures it crystallizes in the cubic system (sphalerite) the more stable form, and at higher temperatures in the hexagonal system (wurtzite) the rarer form. Zinc occurs as zinc blende (sphalerite), nominally zinc sulphide, ZnS, but can have varying proportions of iron or manganese. It is an important ore of zinc often associated with galena, lead sulphide, an ore of lead, and is luminescent and sometimes fluorescent, the fluorescent variety also exhibiting triboluminescence (emits flashes of light when lightly rubbed with a knife or stone). Sphalerite also contains recoverable quantities of gallium, indium and germanium as impurities. Other important ores are zincite, (Zn,Mn)O, a deep-red coloured mass also known as red oxide of zinc, spartalite or sterlingite; zinkosite, an anhydrous sulphate occurring in Spain; and willemite, zinc silicate, Zn2SiO4. Hemimorphite, a hydrated zinc silicate, Zn4Si2O7(OH)2.H2O, is strongly piezoelectric and pyroelectric and can be known as calamine. Calamine is really two different minerals, smithsonite and hemimorphite. Smithsonite, zinc carbonate, ZnCO3, is a typical allochromatic mineral being white when pure, but green with copper impurities, bright yellow with cadmium, pink or violet with manganese and brown with iron oxides, and frequently banded. Zinc spinel, gahnite, is zinc aluminate, ZnAl2O4. Franklinite, a complex zinc manganese iron oxide, used to be an important ore when zinc was alloyed with iron. Hydrozincite, Zn5(CO3)2(OH)6, occurs as a greyish white mass. Rosasite, hydrous copper zinc carbonate, (Cu,Zn)2(CO3)(OH)2, is crystalline green and a minor ore. Other minerals of interest only to collectors are green adamite, Zn2(AsO4)(OH); green aurichalcite, (Zn,Cu)5(CO3)2(OH)6; tarbuttite, Zn2(PO4)(OH); and a bluish green gem mineral phosphophyllite, a hydrated zinc iron manganese phosphate. Zinc occurs as five stable isotopes, 64% zinc-64, 28% zinc-66, 19% zinc-68, the rest being zinc-67 and zinc-70. Altogether, 18 other radioactive isotopes of zinc are known, ranging from zinc-57 to zinc-80. Claim to fame: |
![]()