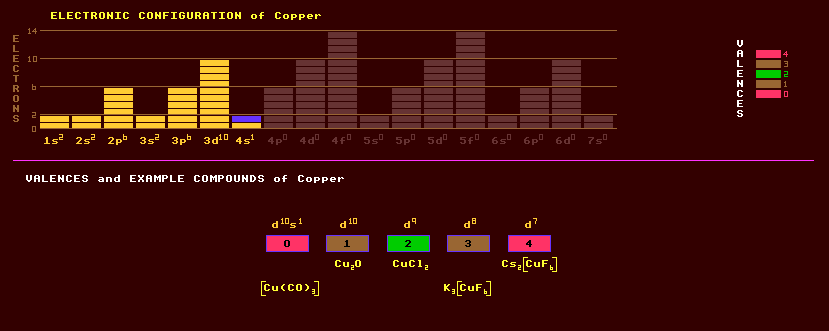

|
29 COPPER Cu (Latin: Cuprum = Cyprus)
Copper is a bright, very ductile, reddish metal capable of being drawn into very thin wire and resists corrosion, though is not immune to attack by atmospheric pollutants, forming an opaque green film of basic copper carbonate, CuCO3,Cu(OH)2, or verdigris, to be found on copper domes atop buildings. Copper is used extensively in electrical wire to conduct electricity because of its very high electrical conductivity, second only to silver, but is much cheaper. Its high thermal conductivity is also slightly bettered by silver, but much bettered by osmium and diamond. Copper can be alloyed with many metals, and is the basis of brass, bronze and aluminium bronze, monel and gun metal. Cupro-nickel is very ductile and used for coinage and condenser tubes. The high electrical conductivity is proffered by the metallic bonding of the copper atoms; the valence electron is allowed free roam throughout the bulk of the metal. This metallic bonding is also responsible for the high ductility of good electrical conductors; and the more metallic the bonding, the less brittle is the substance. Copper oxide, Cu2O, used to be used in copper oxide rectifiers until germanium and silicon were used as semiconductors. Copper sulphate, bluestone or blue vitriol crystals, CuSO4·5H2O, which are azure blue, can be dissolved in water and used to copper plate iron and is a copper ore known as chalcanthite. Copper sulphate is used as an algicide in water purification, and as an agricultural poison. Cuprammonia is a solvent for cellulose. Cuprammonium tetra-amine copper is used in the making of rayon. Copper acetylide, Cu2C2, made by bubbling acetylene gas through an ammoniacal solution of copper chloride, is explosively sensitive to touch when dry. It can also form inside any copper pipes that carry acetylene gas, as used in oxy-acetylene welding. Copper is an essential trace element in life, occurring in many enzymes such as cytochrome oxidase and many other free-oxygen scavenging enzymes, and in haemocyanin, the transporter of oxygen in the blood of arthropods, acting in an analogous way to the iron based heamoglobin in mammals. Excess copper is transported out of the body by a protein called metallothionein, in the same way it transports many other excess metals (like cobalt, another essential trace element). The popular use of copper bracelets to alleviate rheumatism is not supported by any scientific evidence. Copper is toxic to many micro-organisms, copper sulphate is sprayed onto grapes to protect them from fungal infections. A few plants are especially tolerant of high levels of copper in the soil, and accumulate it in large concentrations, possibly sequestering it away out of harms way. Such plants have been used as indicators of extractable deposits of copper minerals in the ground. Phthalocyanine blue, or copper phthalocyanine, is a blue pigment. When halogenated, phthalocyanine green results, which is also used as a pigment and is available in a number of shades varying from deep-blue/green to bright green. Copper is a strongly siderophilic element, many of its minerals are compounds with sulphur, and sometimes with arsenic. Metallic copper objects wrought before 2000BC often contained incidental arsenic, until bronze alloys made with tin were discovered. Important ores of copper are: Chalcopyrite (cupriferrous pyrite), CuFeS2, a copper iron sulphide crystallizing in the tetrahedral system, is the commonest ore of copper and has a metallic brassy yellow colour similar to iron pyrites but richer and often with an iridescent blue and red tarnish. Unlike pyrites, it can be scratched with a knife. Dealers often heat treat chalcopyrite giving it a red/blue iridescence to misrepresent it as bornite, or peacock ore, see below. Cuprite, copper oxide, Cu2O, is usually dark ruby-red, with sometimes octahedral crystals and is associated with native copper, found in Redruth and Liskeard, Cornwall, and also around the World. Chalcotrichite is a red semi-translucent needle shaped variety of cuprite. Tenorite, CuO, or copper black develops on the surface of copper ores as black earthy masses. Important ores include the sulphides covellite, CuS, usually a flat highly iridescent indigo blue, and chalcocite, Cu2S. Others are enargite, Cu3AsS4; and the bronze coloured bornite, Cu5FeS4, which develops an iridescent purple and blue tarnish and for this reason is known as peacock ore (being the true Peacock ore and not another mineral heat-treated to resemble it). Bornite is isometric above 228 Celsius and trigonal below. Cubanite, CuFe2S3 is bronze yellow with a metallic lustre. Berzelianite, Cu2Se, is the rare selenium homologue of chalcocite. Brochantite, Cu4(SO4)(OH)6, is a bright green fibrous hydrated sulphate. Native copper can be found in thin sheets filling rocky fissures and crystallizes in the cubic system. Chalcopyrite, CuFeS2 is the primary mineral that forms a successive series as it is further enriched with copper, proceeding through bornite, Cu5FeS4; covellite, CuS; chalcocite, Cu2S; and (rarely) ending in copper, Cu. Several much rarer higher-order copper sulphides are known: digenite (cubic system), Cu9S5; djureite (monoclinic), Cu31S16; and spionkopite (hexagonal system), Cu39S28. Dioptase, a hydrous copper silicate, Cu3(SiO3)6·6H2O, is vivid emerald in colour and shows outstanding optical dispersion (due to it having a refractive index that varies strongly with wavelength) displaying a wonderful play of colours ('fire'), but it is fairly soft limiting its use as a gemstone. Chrysocolla, hydrated copper silicate, CuSiO3.nH2O, is a secondary mineral occurring as blue/green encrustations on chalcopyrite and other sulphides of copper. Other minerals are: Cupro-uranite, torbernite, Cu(UO2)2·12H2O, a beautiful rich-green radioactive hydrated phosphate of copper and uranium crystallizing in the tetragonal system and usually associated with autenite, a lime green ore of uranium. In the open air, torbernite loses water and turns into meta torbernite with only eight molecules of water of crystallization. Chalcostilbite, wolfsbergite, Cu(UO2)2(PO4)2·12H2O, is a copper antimony sulphide mineral. Others include aurichalcite, (Zn,Cu)5[(OH)3CO3]2; tetrahedrite, Cu12Sb4S13 a tetrahedrally formed grey black copper antimony sulphide and the related but much rarer tennantite, Cu12As4S13 a copper arsenic sulphide of the same form and colour; bournonite, PbCuSbS3; atacamite, Cu2(OH)3Cl; azurite, Cu3(CO3)2(OH)2, a hydrated carbonate and blue ornamental stone once used crushed as the pigment mountain blue (but which gradually turns green into malachite); malachite, Cu2CO3(OH)2, a banded green hydrated carbonate formed by the weathering of copper and copper ores was also once used as a pigment (mountain green) and is still used as a polished decorative stone in ornaments; pseudomalachite, Cu5(OH)4(PO4)2, a dark green hydrated phosphate which develops as a secondary mineral from the alteration of chalcopyrite and other copper ores; turquoise, CuAl6(PO4)4(OH)8·4H2O, a copper aluminium phosphate forming a usually turquoise coloured encrustation, the attractively patterned varieties of which are used as precious stones; connellite, Cu19Cl2SO4(OH)32.H2O a blue chlorosulphate; cyanotrichite, Cu4Al2(SO4)(OH)12.2H2O, a cyan to dark blue coloured copper aluminium sulphate; brochantite, Cu4SO4(OH)6, an emerald green hydrous copper sulphate; there are many others. Copper can also occur in native form by the crystallization of hot solutions or the decomposition of sulphur containing copper ores. The native form usually has encrustations of blue azurite and green malachite. Atacamite, Cu2(OH)3Cl; azurite Cu3(CO3)2(OH)2; malachite Cu2CO3(OH)2 and chrysocolla, CuSiO3.nH2O, were all known to ancient civilisations who roasted the ores with charcoal and a limited supply of air to produced copper oxide, Cu2O, and carbon monoxide, CO, which subsequently reduced the copper oxide to molten metallic copper. Any iron present in the ores would be combined with oxygen to produce fayalite, 2FeO.SiO2, a constituent of the glassy slag which floated on top of the melt. The copper was alloyed with gold or silver to produce harder alloys. Arsenopyrite, FeAsS, was sometimes mixed with the copper ores before smelting to produce very hard and tough but not brittle copper arsenic alloys. Copper exists as a mixture of two stable isotopes, 69% Cu-63 and 31% Cu-65. A further twenty radioactive isotopes are known, ranging from positron emitting Cu-55 to electron emitting Cu-77. Copper-64 can decay by either of the two routes, inverse beta decay or beta decay. Claim to fame: |
![]()