|
28 NICKEL Ni (German: kupfernickel = Devil's copper)
Nickel is a hard, malleable, ductile, silvery white corrosion resistant metallic element able to take a high polish. Nickel is unaffected by alkalis and nitric acid, but dissolves in other acids. Nickel is ferromagnetic at room temperature, with a Curie temperature of 360 Celsius. Small torroids of nickel were used as magnetic core memory in computers. Nickel is also magnetostrictive, shortening in length in a magnetic field.
Mu-metal, an alloy containing 77% Ni, 16% Fe, 5% Cu and 2% Cr, has a high magnetic permittivity and is used as magnetic shielding. Permalloy is similar. Alnico, an alloy of aluminium, nickel and cobalt containing some iron and copper, is used as a high energy permanent magnet. Nichrome, an alloy of nickel and chromium, and Constantan, an alloy of 60% copper and 40% nickel are used as resistance wires because of their resistance to high temperatures, their high resistivity and low-temperature coefficient of resistance. Nichrome is also used in strain-gauges. Inconel and Nimonic, high temperature alloys containing nickel and chromium and other metals, are used for gas turbine blades. Nickel makes stainless steel stainless. Nickel is used for coinage as cupro-nickel and nickel silver (german silver) and is the main constituent of monel metal. Invar, an alloy containing 63.73% iron, 36% nickel 0.2% silicon and 0.07% carbon, has a vary low to zero temperature coefficient of expansion, and is used for pendulums and tuning forks and shadow masks in colour TVs. Nickel is used to nickel-plate steel and brass. Nickel wire supports electrodes in thermionic valves. Shape memory alloy, nitinol, an alloy of nickel and titanium, remembers its previous shape when heated.
Interleaved alternate foils of nickel and aluminium are being used as a new form of welding, where the current from a battery heats the foils initiating an exothermic reaction between the two metals producing a weld, like a modern version of the thermit reaction.
Nickel is extensively used as a catalyst. Finely divided nickel catalyses the hydrogenation of vegetable oils for margarines. Nickel colours glass green and is used in nickel cadmium (NiCd) and nickel iron (NiFe) accumulators. The newer nickel-metal hydride rechargeable cells use electrodes of nickel and manganese-nickel MnNi5, with hydrogen absorbed into the latter. They are superior to NiCd cells in being without the 'memory' effect which can prevent full use of the theoretical capacity in NiCds. Hydrogen is stored within nickel as nickel hydride for use as a fuel in some experimental vehicles. The hydrogen is released on heating the hydride.
Nickel is an essential trace element for life, and is present in urease, the enzyme which catalyses the the decomposition of urea, (NH2)2CO, into ammonia, NH3. In anaerobic bacteria, nickel is coordinated to a tetrapyrrole ring (similar to one coordinated to iron in haem which transports oxygen in blood) within the co-enzyme F-430. The bacteria derive their energy by using the enzyme to metabolises hydrogen, H2, liberating methane, CH4. To many plants, except certain tolerant species which can accumulate high concentrations, nickel is toxic. Dermatitis can be caused by prolonged contact with nickel jewellery in certain individuals who can become sensitised. In humans, nickel dust can cause cancer, especially lung cancer.
Nickel is perhaps the seventh most abundant element on Earth as a whole, but most is concentrated in the core along with iron and cobalt, and much less in the crust. About 10% of Earths' core is nickel. Being strongly siderophilic, nickel occurs as sulphides and oxides.
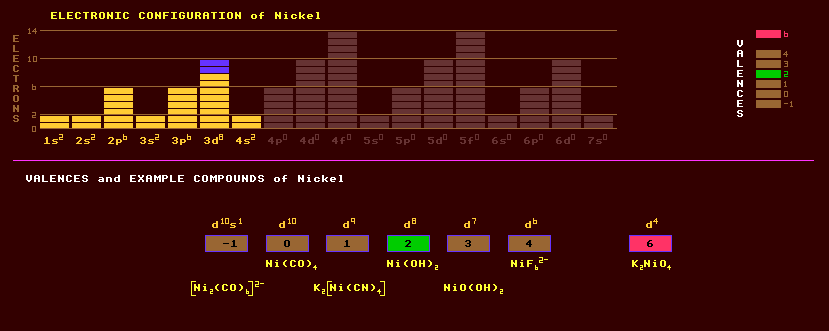
The copper-red nickel sulphide niccolite (nickeline, kupfernickel), NiS; pentlandite, (Fe,Ni)9S8, and pyrrhotite/pyrrhotine or magnetic pyrites, FeS; garnierite, (Ni,Mg)6(OH)8Si4O10(OH)8; and chloanthite, (Ni,Co)As3 are principal ores. Other minerals are ullmanite, a nickel antimony glance, gersdorffite, a nickel arsenic glance, annabergite (nickel bloom), Ni3(AsO4)2·8H2O and millerite, NiS, which appears as radiating flexible whiskers with a brass yellow metallic lustre. Maucherite, Ni11As8, is a reddish silver-white nickel arsenide. Nickel-iron meteorites are an important source of nickel, containing between 7% and 15%, which at room temperature do not form a single mineral, but rather a structural intergrowth of two differing minerals, one with 40% nickel, the other with 5% nickel (kamacite), which produces the characteristic cross-banded appearance of nickel-iron meteorites, called the 'Widmannstatten' structure. Nickel is extracted from its ores in the Mond process where it is first roasted to oxides, any copper is dissolved out with sulphuric acid, the nickel oxide is then reduced to impure metallic nickel by heating with hydrogen. To further purify the nickel, it is reacted with carbon monoxide to form nickel tetracarbonyl, Ni(CO)4, a highly volatile and extremely poisonous substance, which is then decomposed into pure nickel on heating.
Nickel is a mixture of 5 stable isotopes of which nickel-58 is the most abundant at 68%. A total of 18 isotopes are known ranging from nickel-52 to nickel-69. The radioactive positron emitter, Nickel-56 is made in huge amounts in Supernovas by the irradiation of the ejected iron-56 from the outer layers of the star by the intense 10 second pulse of neutrinos emitted during the collapse of the core of the star when becoming a neutron star. Nickel-56 decays by inverse beta decay with a halflife of 6 days into cobalt-56, itself a positron emitter with a halflife of 77 days. The subsequent decay of cobalt-56 by inverse beta decay back into iron-56 is responsible for the characteristic 77 day decay in luminosity of type II supernovas.
In the year 1999 the most proton-rich nucleus observed so far was created. That is, one with the highest proton:neutron ratio. This nucleus, nickel-48 is doubly magic, with both a magic number of neutrons (20) and a magic number of protons (28). This makes it comparitavely stable compared to its surrounding nuclides, with less mass than nuclides in its neighbourhood. It is thus thought incapable of mere proton decay because the resulting nucleus of cobalt-47 would contravene energy conservation, and must instead decay by the unique double-proton decay into iron-46. Although nickel-48 is comparatively stable due to being doubly magic, because it is situated very far from the valley of stability, it has only a very short halflife, about half a microsecond.
Claim to fame: Nickel exhibits the highest temperature coefficient of resistance, 6900 ppm/ºC.
|

![]()
![]()