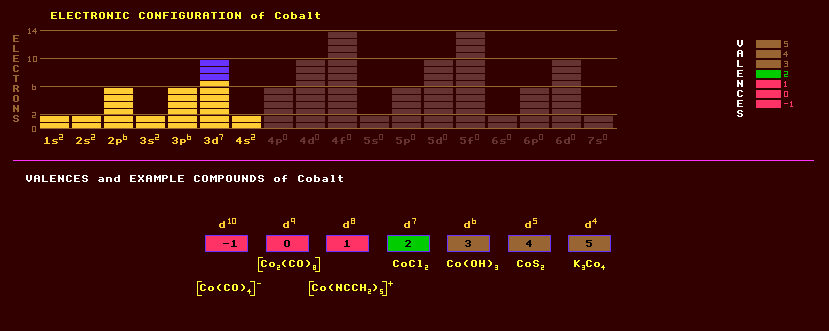

|
27 COBALT Co (Greek: cobalos = mine)
Cobalt is a hard grey metallic middle transition element belonging to the ferrous-nine group and is ferromagnetic below 1075 Celsius, with a magnetic permeability about 2/3 that of iron. Cobalt can take a high polish and has a high tensile strength. Cobalt exists in two allotropic forms over a wide temperature range, the beta form predominating below 400 Celsius, the alpha form above, but the transformation is very slow and accounts for the widely different reported properties of cobalt. Cobalt is similar in properties to iron or nickel and is used extensively in alloys. Cobalt steels contain 5-20% cobalt, 14-20% tungsten, 4% chromium, 1-2% vanadium, 0.8% carbon and a trace of molybdenum have great hardness and used for cutting but are brittle. Stellite alloys contain cobalt, chromium and tungsten, again used for high speed and high temperature cutting and for dies. Alloyed with aluminium and nickel it forms Alnico used as a permanent magnet of great magnetic strength. Cobalt chloride, CoCl2·6H2O, is strongly deliquescent and is used as a moisture indicator in the desiccant, silica gel, where it turns colour from a deep azure blue when devoid of water of crystallization to a pinky red when saturated. Cobalt black, or cobaltic oxide, is a black pigment used in heat resistant paints and in pottery. Cobalt blue or Thenards blue is essentially cobalt aluminate, CoO.Al2O3, a very stable pigment of low tinting strength used since 1802. Cobalt oxide, CoO, is used to produce a deep blue colour in glass, and in smaller quantities to counteract the slight greenish tinge in glass due to iron impurities. Cobalt violet is a pigment obtained by reacting cobalt salts with alkaline earth phosphates. Cerulean blue or cobaltous stannate, CoO.SnO2, is a light blue pigment introduced in the 1800's which is greener in tone than cobalt blue. Cobalt is an essential mineral contained in vitamin B12, cyanocobalamin, and is manufactured in small amounts from dietary cobalt by bacteria in the human colon. Some other bacteria and fungi produce a methylated variety of vitamin B12, which can donate the methyl group to other unwanted metals such as mercury, tellurium, selenium or arsenic in their diet to produce highly volatile derivatives, thus ridding themselves of potentially poisonous metals. But the bacteria themselves are also highly toxic. Cobalt is a strongly chalcogenic and siderophilic element that is probably concentrated in the Earth's core along with iron, where it is inaccessible. Cobalt also occurs in the crust as erythrite or cobalt bloom, a deep magenta-red cobalt arsenide, Co3(AsO4)2·8H2O, and in cobaltite, CoAsS; glaucodot, (Co,Fe)AsS; and skutterudite, (Co,Ni)(As2,As3); linnaeite, Co3S4; and smaltite, (Co,Ni)As2-3. It is often associated with nickel, lead, silver, copper and iron ores from which it is frequently obtained as a byproduct. Other minerals are the rose red sphaerorocobaltite (cobalticalcite), CoCO3 and heterogenite, CoOOH. Cobaltiferrous wad is an impure hydrated oxide of manganese containing up to 30% manganese. Cobalt vitriol, or bieberite is hydrated cobalt sulphate, found as stalactites and stalagmites in old mines containing other cobalt minerals. Cobaltomenite is a hydrated cobalt selenide. Cobalt-60 is a dangerously radioactive high energy gamma ray emitter thousands of times more intense than radium and has a halflife of 5.3 years. It is a beta decayer that also emits unusually energetic gamma rays of 1.3MeV energy. It is used in the radiographic treatment of cancer and to sterilise foodstuffs by irradiation. Cobalt-59, the only stable isotope of cobalt, is used in the cobalt bomb, a nuclear bomb designed not to damage many buildings but to kill people by radiation instead. The cobalt-59 surrounds a small (less than 1 kiloton equivalent) thermonuclear bomb and absorbs neutrons to become cobalt-60, the dangerously radioactive product designed to kill people. Sixteen other radioactive isotopes are known, from Co-50 to Co-66. The radioactive positron emitter, Nickel-56 is synthesised in copious amounts in Supernovas by the irradiation of the ejected iron-56 from the outer layers of the star by the intense 10 second pulse of neutrinos emitted during the collapse of the core of the star into becoming a neutron star. Nickel-56 decays by inverse beta decay with a halflife of 6 days into cobalt-56, itself a positron emitter with a halflife of 77 days. The subsequent decay of cobalt-56 by inverse beta decay back into iron-56 is responsible for the characteristic 77 day decay in luminosity of type II supernovas. Claim to fame: Cobalt has the highest Curie temperature of any ferromagnetic element (1484K).
|
![]()