
|
26 IRON Fe (Latin: Ferrum)
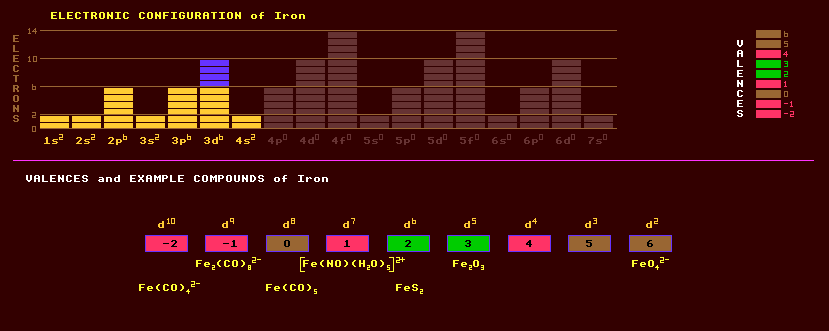
Iron is a lustrous silvery white soft, malleable transition metal which rusts in damp air and is dissolved by dilute acids. Rust catalyses irons own rusting. Iron exists in four polymorphic forms: alpha-iron is on a body-centred cubic lattice stable below 906 Celsius and ferromagnetic below the Curie temperature of 768ºC and non magnetic between 768ºC and 906ºC when it is known as beta iron; gamma iron is stable between 906ºC and 1403ºC, a face-centred cubic known as austenite in solid solutions; and delta iron which is face-centred cubic (the same as alpha-iron) between 1403ºC and the melting point 1532ºC. Iron is ubiquitous throughout the Universe because its nuclei are the most stable and have the highest binding energy per nucleon. Iron is the fourth most abundant element in the Earths' crust. The Earths inner core is thought to be made of nickel iron, the outer core being of dense iron sulphides. Carbon is added to iron in increasing amounts to make first soft wrought iron, then tough steel, then brittle cast iron. Pig iron contains 3% carbon and other impurities. Iron is used in alkaline nickel-iron accumulators (NiFe cells). Magnetic uses of iron abound: Soft iron for solenoids, silicon-iron for low loss transformers, non-conducting ferrites (compressed and sintered oxides of iron and other metals) for tape heads, ferrite rod aerials, transformers, dust cores, and magnetic tape coatings. Low quality wire recorders used iron wire to record sounds before tape was invented, but now it is back in the form of iron coated plastic tape for the highest quality recordings. Permanent magnets are made mostly of iron alloys, an alloy with neodymium and boron, Nd2Fe14B, having the highest energy product of any known permanent magnet (50 Mega GaussOersteds). Iron alloy metallic glasses where the molten alloy has been cooled so rapidly that crystallization had no time to occur exhibit very small hysteresis loss. For instance, METGLASS 2605S-2, Fe79B13Si9, has lower hysteresis loss than the best grain-oriented silicon steels. Three oxides are known: Ferric oxide, FeO, ferrous oxide, Fe2O3, and ferroso-ferric oxide, FeO.Fe2O3. Ferroso-ferric oxide is ferromagnetic with a room temperature magnetisation of 401 Gauss and a Curie temperature of 300 Celsius and is used as the magnetic coating of ferric audio recording tapes. Iron forms complexes. Iron tetracarbonyl, Fe(CO)4 is a gas and iron pentacarbonyl, Fe(CO)5 is liquid. Ores of iron include the strongly magnetic magnetite, Fe2O3.FeO, which forms shiny black octahedral crystals often found as black sands along beaches and the banks of streams (also known as lodestone, used as a primitive compass needle); ; haematite, Fe2O3, the most common ore, is rhombohedral, black and often botryoidal but sometimes like a bowl of cornflakes; siderite, iron carbonate, FeCO3 a pale yellow to dark brown mineral; goethite, iron hydroxide, alpha-FeO(OH) an important constituent of limonite (a term used for a mixture of oxides and hydroxides occurring in a multitude of forms); the golden yellow 'fools gold' or iron pyrites, FeS2, which forms dodecahedral, pyritohedral or cubic shaped crystals. Iron pyrites, or pyrites, is so named from the sparks which fly off when broken. Pyrite weathers and oxidises very easily tainting the surface a vivid red, green or blue and releasing sulphuric acid with obvious implications when stored in collections. Marcasite and pyrite are polymorphs of FeS2 crystallizing in the orthorhombic and cubic systems respectively; with pyrite being the most dense, both are brassy yellow with a metallic lustre and are sources of sulphur and both can be found in coal. Pyrrotite, Fe7S8, also brassy yellow, is magnetic and has a slightly variable amount of iron, examples with a slight excess of iron crystallize in the hexagonal system, whereas those with more sulphur in the monoclinic system; all become hexagonal when heated to 350 Celsius. Pyrrotite and marcasite tend to crumble and decompose to sulphur and iron in collections. Arsenopyrite, FeAsS, is the same as pyrite but with an atom of arsenic in place of one of the two atoms of sulphur. Cubanite, CuFe2S3 is bronze yellow with a metallic lustre. Troilite, FeS, is closely related to pyrrotite but is non-magnetic and found in some nickel-iron meteorites. Niningerite is a magnesium iron sulphide not found on Earth but rather in oxygen-poor minerals present in chondritic meteorites. Prussian blue, or iron hexacyanoferrate, Fe4[Fe(CN)6]3, was introduced as a blue pigment in 1704 and is also know as berlin blue. Bog iron ore is formed by the iron bacteria which converts iron oxide to iron hydroxide in bogs. Ochre or yellow ochre is a mixture of iron oxides and silicates used as a natural pigment. The Earths centre is thought to be liquid iron with perhaps 10% occluded hydrogen. Iron also occurs in basalt rocks. Iron is purified in blast furnaces or Bessemer converters by blasting air into a burning mixture of coke, limestone and iron ore. Iron has four stable isotopes, of which iron-56 is the most stable and most abundant at 92%, followed by iron-54 at 6%, iron-57 at 2% and 0.3% of iron-58. Twelve other radioactive isotopes are known, ranging from iron-48 to iron-64. Claim to fame: Iron has the highest terrestrial abundance of any element and the highest binding energy per nucleon (8.6MeV/nucleon).
|
![]()