
|
25 MANGANESE Mn (Latin: magnes=magnet)
Manganese is a brilliant white, hard, brittle metallic element with a reddish tinge. It exists in four allotropic forms, Manganese is used to make steel, and in the making of silico-manganese steel, aluminium-bronze, and aluminium and nickel based alloys. Manganin, a copper based alloy with manganese and nickel is used to make electrical resistance wire with a low temperature coefficient of resistance. Manganese can display a great variety of valences in its compounds from one to seven, which are mainly deeply coloured. Manganese compounds colour borax beads deep purple to black. Manganese will colour glass amethyst and is also responsible for the colour of true amethyst, an aluminium oxide. Several aluminium manganese alloys, amongst them Al4Mn, Al6Mn, Al6Li3Cu and Al28Cr1Ru5, 'crystallize' in the forbidden pentagonal symmetry, which is halfway between a glass and a periodic lattice. The quasi-crystal is based on an icosahedral unit of 12 atoms of aluminium surround a smaller manganese atom, which nearly forms a sphere, but not quite since there is nearly room for a thirteenth atom. Then 12 of these near spheres pack around a central near-sphere forming another near sphere, and so on hierarchically. Another, Mg32(Al,Zn)49 consists of two basic space-filling rhombohedral cells that are joined haphazardly (similar to the darts and wedge parallelograms of the Penrose tiling patterns for the plane, which cannot be arranged with any periodic order).
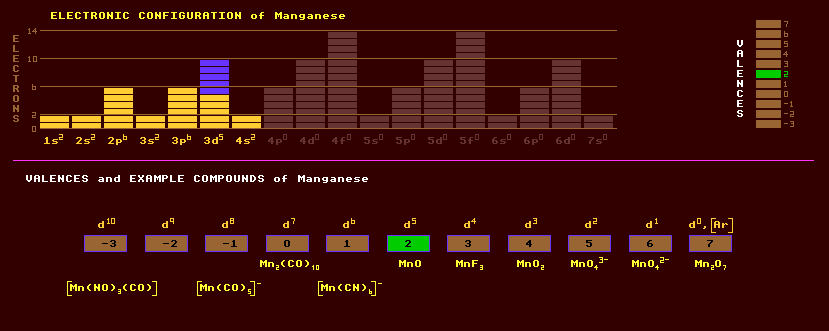
Manganese dioxide is a soot-black powder used in the laboratory to generate oxygen gas, and as a depolarizer in dry cells, where any generated hydrogen and oxygen must be quickly recombined to make water, otherwise the cell will explode. Manganese heptoxide, Mn2O7, is an acidic, heavy, dark coloured oil and strong oxidizing agent which is explosively unstable and forms manganates. Mangano manganic oxide, Mn3O4, is a red neutral mixed oxide. Manganic oxide, Mn2O3 is a brown powder occurring as braunite. Manganous oxide, MnO, a basic oxide occurring as Manganosite, crystallizes in the cubic system. Manganese carbide, Mn3C, liberates methane gas, CH4, on contact with water, forming manganous hydroxide, Mn(OH)2. The deep purple potassium permanganate, KMnO4, with a bitter-sweet taste, is used as an oxidant and antiseptic. Blue or blue-green hypomanganates, M3MnO4, are formed by heating the green manganates, M2MnO4, with alkali hydroxides. Some manganese compounds, in particular MnAs, MnBi and MnSb are ferromagnetic with room temperature magnetizations of 670, 620 and 710 Gauss, and Curie temperatures of 45ºC, 357ºC and 314 Celsius respectively, which for the most part are higher than those for chromium dioxide of 515 Gauss and 113 Celsius. The principal ores of manganese are pyrolusite, MnO2; rhodochrosite, MnCO3 a rose pink carbonate; psilomelane, BaMn9O16(OH)4; rhodonite, CaMn4(SiO3)5, a pink to red silicate of manganese and calcium which turns black on weathering; and hausmannite, Mn3O4. Other minerals include manganite, MnO(OH), an important steely grey to black ore. Manganese is recovered from the ore pyrolusite, MnO2, by mixing it with aluminium powder and igniting the mixture as in the thermite reaction. Tephroite, Mn2SiO4 is an orthorhombic manganese silicate. Just one stable isotope of manganese exists, manganese-55. The positron emitter, manganese-53, produced in the upper atmosphere in trace amounts by cosmic ray bombardment of dust, has a halflife of 3.7 Myr. Claim to fame: Manganese exhibits the highest electrical resistivity of the metallic elements, 185microOhm centimetre and also displays the greatest number of valences (11).
|
![]()