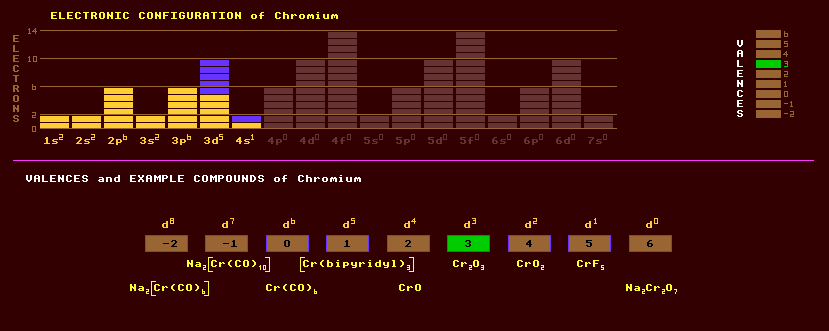

|
24 CHROMIUM Cr (Greek: chroma = colour)
Chromium is a steel-grey lustrous white metal that can take a high polish. In contrast to iron cobalt nickel and gadolinium, which are all ferromagnetic at room temperature, being well below their respective Curie temperatures, chromium is the only element which is anti-ferromagnetic at room temperature, which means adjacent atoms electron spins are aligned in anti-parallel orientations, provided it is below the Neel temperature, Tn, which for chromium is 308 Kelvin, or just 35 Celsius. Chromium, being one of the transition metal elements, produces many colourful salts. The dichromates are powerful oxidizers. Ammonium dichromate, (NH4)2Cr2O7, an orange crystalline salt, burns producing a voluminous dull green powder, chromium sesquioxide, Cr2O3, also known as chromic oxide, sometimes used as a pigment. Potassium dichromate, K2Cr2O7, which is also orange, is used to tan leather, as in chrome leather. Chromic acid, H2CrO4, is the aqueous solution of chromium oxide, CrO3, a poisonous, deliquescent and powerful oxidant. Lead Chromate, Pb2CrO4, is used as the yellow pigment chrome yellow. Other chromates may be green or violet, and are quite stable. Chromium is an essential trace element used in the metabolism of glucose. Chromium deficiency can lead to late-onset diabetes and hardening of the arteries. In the much more soluble chromate form it is toxic and carcinogenic. Chromium is electroplated onto steel with the help of potassium ferricyanide to produce a shiny, hard, corrosion resistant finish. Aluminium is anodized by electrolysis in a solution of chromic acid. Steel alloyed with chromium produces stainless steel, which does not rust and is non-magnetic, added molybdenum, produces chrome molybdenum steels, used in screwdrivers. alloyed with nickel it produces nichrome, used as resistance wire of high melting point. Crystalline needles of chromium dioxide, CrO2, are ferromagnetic, with a Curie temperature of just 113 Celsius, and is used as the magnetic coating of chromium dioxide compact cassette audio recording tapes. The magnetisation is higher than that of the ferrosoferric oxide, FeOFe2O3 used in ferric tapes which measures 480 Gauss. Chromium is used to colour glass emerald green, and is used as the dopant added to synthetic ruby crystals made of alumina to make ruby lasers, which emit red light. Its principal ore is chromite, (Fe,Mg)O.Cr2O3, in the form of grey-black octahedral crystals, and is a double oxide of chromium and iron. Chromium was first extracted from the orange-red crystals of crocoite, lead chromate, PbCrO4, a secondary mineral resulting from the alteration of other lead minerals by hydrothermal fluids. Chromium is extracted commercially by heating the ore with silicon or aluminium. Rather high concentrations of chromium and nickel are to be found in 'serpentine soils', derived from the weathering of ultrabasic rocks (those with a low silica content). These soils are toxic to most forms of life, supporting only tolerant vegetation. Chromium consists of four stable isotopes, chromium-52 being the most abundant at 84%, followed by chromium-50, -53, and chromium-54. Claim to fame:
|
![]()