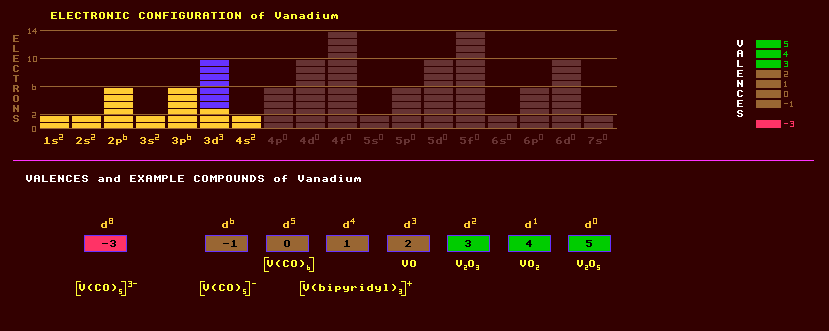

|
23 VANADIUM V (Vanadis = Scandinavian Goddess)
Vanadium is a very hard whitish grey, soft and ductile when pure, transition metal element named after the Scandinavian goddess, Vanadis, because it has multi-coloured compounds. Vanadium is used to harden steels, sometimes used in conjunction with chromium, as in chrome vanadium screwdrivers. The vanadium removes occluded oxygen and nitrogen thus improving tensile strength. Manganese vanadium steel alloys are also used in high speed tools. Vanadium has good corrosion resistance to acids and alkalis but oxidises above 660 Celsius. It has a low neutron capture cross sectional area and is used in nuclear plants. Vanadium is found in over 65 minerals, the most important being the lead-grey patronite, VS4; the yellow-green carnotite, a hydrated potassium uranium vanadate, K2(UO2)2(VO4)·H2O; roscoelite; and the yellow to ruby-red vanadinite, lead vanadate chloride, Pb5(VO4)3Cl. Other minerals include tyuyamunite, Ca(UO2)2(VO4)2·5-10H2O, which fluoresces yellow under ultraviolet light unlike carnotite; francevillite, (Ba,Pb)(UO2)2(VO4)2·5H2O; and descloizite, Pb(Zn,Cu)VO4(OH). It is also present in phosphate rock and crude oils, and is also recovered from the sediments from bauxite processing. It can be produced by reduction of vanadium trichloride with magnesium in an atmosphere of argon, or by reducing the oxide V2O5, with calcium. Vanadium pentoxide is used in ceramics, as a catalyst in the production of sulphuric acid, as a mordant in dyeing and in making aniline black. Vanadium and its compounds are toxic. Vanadium occurs as a mixture of two isotopes, 99.8% of the stable vanadium-51, and just 0.2% of the slightly radioactive vanadium-50, which has an enormous halflife of 140,000 million million years (far greater than the age of the Universe) and can decay by either beta decay or inverse beta decay.
Claim to fame: vanadium has the highest spectral emissivity of any metallic element (0.65 @ 0.65
|
![]()