
|
22 TITANIUM Ti (Titans - sons of the Earth Goddess)
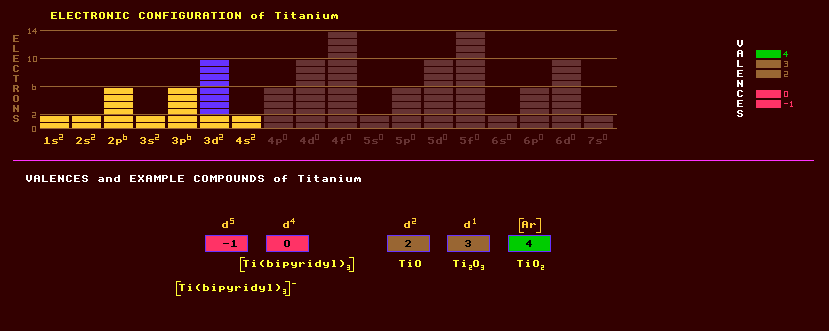
Titanium is a strong white corrosion-resistant metal resembling iron but nearly half as dense and the ninth most abundant of the Earths elements. It is ductile only if free of oxygen. Titanium is widely used as a strategic metal in aircraft such as for the skin in the Lockhead SR-71 Blackbird because of its lightness and resistance to heat generated by flying at Mach 3. It can be added to stainless steel to further increase corrosion resistance. It is resistant to sea water and is used on ships. It exists in two allotropic forms, the alpha hexagonal form very slowly transforms into the cubic beta form when heated above 880 Celsius. Titanium is used to make titanium jewellery, which is highly iridescent, with the colours being dependant upon the thickness of the electroplated film of titanium dioxide, which can be controlled by selecting the applied voltage. Titanium is the only element to burn in nitrogen, forming the very hard titanium nitride, TiN, which is deposited in several layers on the external metal parts of some watches as a hard-wearing golden-coloured protective layer which looks like gold. Thin alternate layers of silicon dioxide (low refractive index) and titanium dioxide (high refractive index) are used to coat photographic and spectacle lenses to both reduce light reflection from air-glass interfaces, and to increase optical transmission. Previously, magnesium fluoride or cryolite (magnesium aluminium fluoride) were used, but they are too soft. Many titanates (and niobates and tantalates and some zirconates and hafnates) crystallize in the anisotropic perovskite structure and generally exhibit a range of active phenomena. Thus lead titanate, PbTiO3, is both strongly piezoelectric and ferroelectric with a Curie temperature of 492 Celsius, see lithium niobate under niobium. Barium titanate, BaTiO3, a ceramic which exhibits a very high dielectric constant, is used to enhance the capacitance in ceramic capacitors and also in ceramic pickup cartridges to replace Rochelle salt, because it has a higher Curie point (135 Celsius). The metal burns in air producing titanium dioxide, TiO2. Titanium dioxide, titania, by virtue of its very high index of refraction of 2.7 which makes it appear dazzlingly white, is used as a pure white pigment of great opacity in paints, plastics and also in foodstuffs like cakes, although Mothers never put this in! Titanium dioxide is used as one of the anti-reflective coatings on multi-coated camera lenses etc. Titanium dioxide is also a good photocatalyst able to oxidise organic compounds when exposed to sunlight. The moon contains much more titanium than Earth, moon dust comprises 12% titanium dioxide. The titanium rocks on the moons surface trap helium-3 ions streaming through outer space driven by the solar wind, which is also much more abundant on the moon than on Earth. See helium. Titanium dioxide can be produced as large crystals for use as a gemstone, it has a higher refractive index than diamond, but is much softer. Compounds of titanium are used as yellow or yellow-red glazes on ceramics. Titanium tetrachloride, TiCl4, is used to iridize glass, and also as a smoke screen, as it fumes strongly in air. Titanium is purified by heating titanium tetrachloride with magnesium in an atmosphere of argon. Titanium does not occur in its native form, but within the minerals rutile, alpha titanium oxide, TiO2; sphene or titanite, CaTi(O)SiO4; and ilmenite, an iron titanite oxide, FeTiO3. Other minerals include perovskite, calcium titanate, CaTiO3. Titanium dioxide is found in three different polymorphic forms: rutile, alpha titanium oxide which crystallizes in the tetragonal system as does the much rarer anatase or beta titanium oxide; and brookite, gamma titanium oxide, which is orthorhombic. Rutile has a refractive index of 2.5, higher than that of diamond, 2.4. Some deposits of titanite are valuable sources of rare earth elements. Titanium exists as five stable isotopes, the most abundant of which is titanium-48 at 74%, the rest comprising titanium-46, -47, -49, and titanium-50. Titanium is reported to become very radioactive after being bombarded with deuterium ions, emitting positrons and gamma rays. Claim to fame:
|
![]()