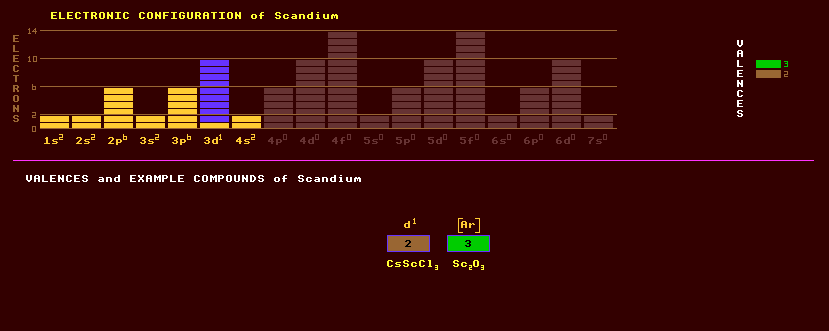

|
21 SCANDIUM Sc (Scandia = Scandinavia)
Scandium is a transition metal element that more closely resembles yttrium and other rare Earths than titanium. It is less basic than the rare Earth metals. It is silvery white, which tarnishes to a pinkish yellow in air, and is fairly soft and difficult to separate from other like metals. It a very light metal but with a higher melting point than aluminium, and thus finds use in space missiles. It was formerly named ekaboron by Mendeleev and first found in Scandinavia, hence its name. It is found in association with yttrium and cerium in gadolinite, or with tungsten in wolframite, (FeMn)WO4, with cerium in allanite, orthite or cerite, with yttrium in thortveitite and with other rare Earths in euxenite, in which it was first discovered. It is widely distributed across Earth in over 800 minerals, but at low concentrations. It forms very few minerals itself. Scandium is more far abundant in the sun and other stars than on Earth. Aquamarine, a bluish type of beryl, is said to be coloured by traces of scandium. Scandium iodide, ScI3, is used as a white glowing phosphor in cathode ray tubes and natural sunlight fluorescent lamps. Scandium oxide, Sc2O3, is used in high-intensity electric arc lamps. The Sc3+ ion forms scandium hydroxide, Sc(OH)3, which is very insoluble in water at neutral pH, and therefore dissolved concentration is very low. It is now obtained from davidite as a by product of uranium extraction, or from the very rare thortveitite, (Sc,Y)2Si2O7, in which it can occur at up to 34%. Scandium exists as just one stable isotope, scandium-45. A total of 12 radioactive isotopes of scandium are known, ranging from the tri-modally decaying scandium-40 which can decay by alpha, proton, or positron emission, to the beta decaying scandium-52. Claim to fame:
|
![]()