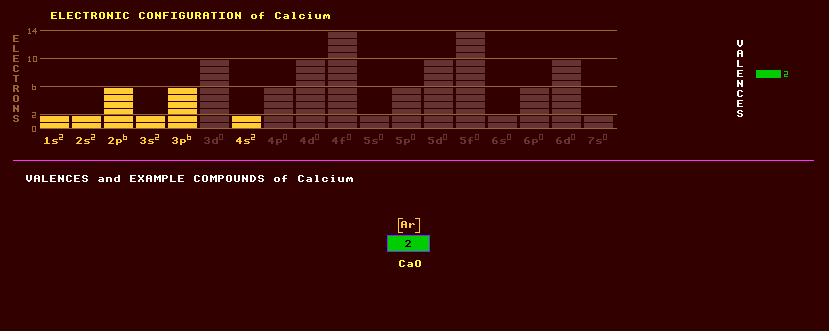

|
20 CALCIUM Ca (Latin: calx = lime)
Calcium is a soft, malleable, white and reactive alkali metal that oxidised when exposed to air and reacts with water to produce calcium hydroxide, Ca(OH)2, which is alkaline. Calcium burns with a red flame to produce calcium oxide, lime, CaO, otherwise known as quicklime, burnt lime, caustic lime, unslaked lime and anhydrous lime. Calcium oxide is used to neutralise acid soils in farming, and in the making of cement and mortar. Calcium oxide is strongly thermoluminescent, giving out a brilliant white light when heated. Limelight was produced when lime is heated by an oxyhydrogen flame. Calcium is used as a getter (of oxygen) in low noise thermionic valves. Calcium does not occur in its native form, but is available in abundance as calcium carbonate in the form of calcite, aragonite, chalk, limestone, marble or dolomitic limestone rock. Most of these formed by the deposition of diatomic skeletons in the warm shallow seas over aeons. Calcite, a common mineral, is a clear crystalline form of calcium carbonate. Of all the minerals, calcite exhibits by far the greatest variety of different crystal forms, appearing as rhombohedra, prisms and scalenohedra, frequently twinned; over 500 different crystal shapes have been identified. The rhombohedral form is known as Iceland Spar, which exhibits strong birefringence or optical anisotropy; the refractive index differs for differing directions, splitting the rays from an object into two and plane polarising them in orthogonal directions, such that two images of an object can be seen through it. Icelandic Spar rhombohedra are used as polarisers in optical instruments. Iceland Spar exhibits perfect cleavage. When pure, calcite is colourless or white, but can be coloured by trace impurities; manganese produces pink calcites, cobalt purple-violet; many are intensely fluorescent under ultraviolet light. Aragonite (orthorhombic), vaterite (hexagonal), calcite (trigonal) and possibly elaterite (a high temperature and high pressure phase) are polymorphs of calcium carbonate, sharing the same chemical formulae but differing in crystal structure and other properties. Unlike calcite, aragonite has very poor cleavage and does not form rhombohedral crystals. Crystallization occurs from hot solutions, calcite forms at lower temperatures, aragonite at higher. Dolomite, calcium magnesium carbonate, CaMg(CO3)2, of which whole mountain ranges can be made, resembles calcite and magnesite in crystal shape and colour and crystallizes in the same rhombohedral system. Calcium carbonate is insoluble in water, unless carbon dioxide is present, with which it form calcium hydrogen carbonate, CaHCO3, which is responsible for hard water. Lime is obtained by burning charcoal with calcium carbonate in a lime kiln which drives off carbon dioxide and water. Anhydrite, calcium sulphate, CaSO4, is formed from sea water by evaporation in a dry climate as does gypsum, hydrated calcium sulphate, CaSO4·2H2O, with which it is associated. Gypsum is very soft, can be scratched with the fingernail and is used in making plaster of Paris and plaster-board for building. When finely divided, gypsum is known as alabaster, when fibrous selenite. When pure, gypsum is white, but can be tinted yellow or brown by impurities. Calcium carbide, Ca2C2, is a hard yellowish black lumpy rock obtained by fusing lime and coal in an electric furnace. It has an energetic triple bond between the carbon atoms, and reacts with water evolving acetylene gas, C2H2, and forming calcium hydroxide, Ca(OH)2. The acetylene gas so obtained is used in cavers lamps, and was used in bicycle lamps. Calcium tungstate is luminescent when irradiated with electrons in a CRT. Calcium chloride, CaCl2, is used as a desiccant for drying air. Calcium fluoride crystals, CaF2, or fluorite, are greeny yellow and/or blue, and emit light when gently heated (thermoluminescence). Under ultraviolet illumination fluorite fluoresces blue, hence the word fluoresce and are used as sub-atomic particle detectors in high energy physics. Derbyshire fluorite is called 'Blue John' and can be carved into ornaments. Egyptian blue, CaO.Al2.4SiO2, was used as a blue pigment as long as 4000 years ago. A different book says that Egyptian blue is a man made version of the extremely rare natural mineral cuprorivaite, or calcium copper silicate, and was made 4000 years ago (and the recipe then lost to modern civilisations until recently) by firing quartz sand with lime, a little alkali and a copper ore at between 850C and 1000C. Thus one book says it contains aluminium not copper, and the other vice versa, I wish they would make up their minds! Calcium occurs as a mixture of six different isotopes, 96% of which is calcium-40, the rest being calcium-42, -43, -44, -46, and calcium-48. Claim to fame: Calcium has the lowest electron affinity (-186KJ/mol) of any element.
|
![]()