
|
19 POTASSIUM K (Latin: Kalium)
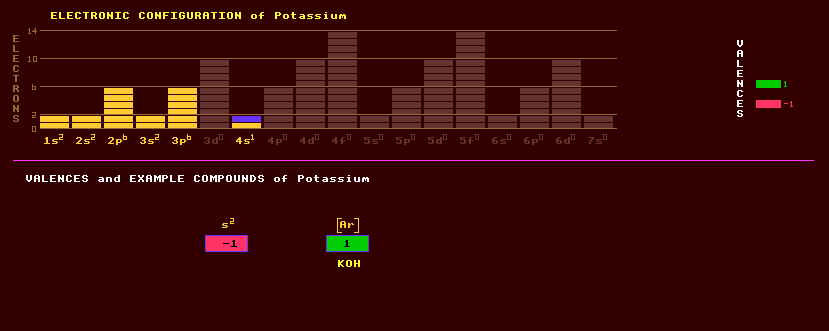
Potassium is a reactive alkali metal which burns with a lilac flame. It quickly oxidizes in air and busts into flame if put in water, so must be stored under oil. It is soft and easily cut with a knife when it exhibits a silvery appearance which then rapidly oxidises. It decomposes in water forming the strongly alkaline potassium hydroxide, KOH, from which it is commercially obtained by hydrolysis. Potassium is also obtained by liberating it from KCl by reduction with sodium. The element itself has little practical use but its compounds are used extensively. Potassium nitrate, KNO3, or saltpetre, is used as a fertiliser and as an oxidant in fireworks and gunpowder; potassium nitrite, KNO2, is used as a preservative for tinned meats despite being linked with cancer risks; potassium cyanide, KCN, is extremely poisonous and used in the extraction of gold and in photographic processing; potassium chlorate, KClO3, is used as the oxidiser in match heads and fireworks; potassium ferricyanide, K2Fe(CN)6, is used in dyeing and etching. Potassium permanganate, KMnO4, is a dark purple strongly oxidizing agent used as a disinfectant. Potassium compounds are widely found in nature. Potassium carbonate, K2CO3, potash, is in the ash from burnt plants. Potassium is essential for plant growth, and soluble potassium salts such as potassium nitrate and potassium carbonate are used extensively as fertilisers. Potassium is also essential to mammals in maintaining osmotic balances and for nerve signal propagation. Potassium superoxide, KO2, is used to re-generate oxygen gas from exhaled carbon dioxide in breathing masks, submarines and manned space satellites. Potassium carbonate, K2CO3 is the end result of this reaction. Potassium metal can be alloyed with sodium, producing NaK, which reduces its melting point to below that of room temperature, and is used as the coolant for some nuclear reactors. Potassium dihydrogen phosphate, KDP crystals, is both piezoelectric and birefringent and is useful as a non-linear medium for optical second harmonic generation by laser light. Potassium forms many evaporite deposits such as the bitter-tasting schoenite or picromerite, K2Mg(SO4)2·6H2O; sylvite, KCl; silvinite, (Na,K)Cl; carnallite, KMgCl3.6H2O; polyhalite, K2Ca2Mg[SO4]4·H2O; from which it can be obtained. Potassium is never found in native form because it is one of the most reactive and electropositive metals. But most potassium occurs in silicate minerals such as the alkali feldspars, (Na,K)AlSi3O8. Potassium nitrate, KNO3, is used as an oxidant in gunpowder and fireworks. Potassium cyanide, KCN, will dissolve gold and is used in it's extraction, but is extremely poisonous. Potassium ferricyanide, K3[Fe(CN)6], is used in chemical analysis, dyeing, etching and blue print paper. Potassium carbonate, K2CO3, or potash, is used in washing, soft soaps, pharmacy and as a glass flux. Potassium chlorate, KClO3, detonates with heat and is used in match heads, fireworks, explosives, and as a laboratory source of oxygen. Potassium perchlorate, KClO4, is also used as the oxidant in fireworks but is more stable and hence safer. Potassium chloride, KCl, is used in pharmacy and photographic development. Potassium bromide, KBr, potassium sulphate, KSO4, potassium chromate, K2CrO4 and potassium dichromate, K2Cr2O7 are other important compounds. Potassium exists as a mixture of two stable isotopes, 94% potassium-39 and 6% potassium-41, and one radioactive isotope, potassium-40 present at 0.02%. Potassium is the first element in the periodic table to be naturally weakly radioactive due to the presence of potassium-40, a long lived isotope with a halflife of 1260 Million years which decays by either beta decay, emitting electrons to become the stable argon-40; or by inverse beta decay emitting positrons, to become the stable calcium-40. The ratio of argon-40 to potassium-40 in Earths rocks is used to date rocks. The calcium-40 produced from the radioactive decay of potassium-40 cannot be used to date rocks because it cannot be differentiated from that normally present in most rocks anyway. The decay of potassium-40 is thought responsible for Earths' geothermal heat flux, which on average, is 60 milli Watts per square metre. The heat flux is responsible for the Earths' increase in temperature with depth, without which it would have long since cooled. Compare this geothermal flux with the solar flux at the Earths' equator of 1350 W/m2 (at noon). Potassium-40 is created by cosmic ray bombardment. Claim to fame: Potassium has the lowest electronegativity, 0.9 units (Pauling).
|
![]()