
|
18 ARGON Ar (Greek: argos = inactive)
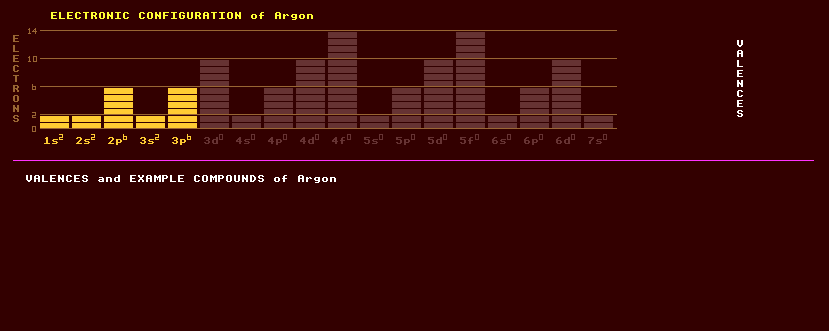
A colourless, odourless noble monatomic gaseous element thought to be completely inert due to its completed-octet electron shell, hence its Greek name, argon, meaning inactive. It is present in air at a high 0.9% (by volume), the next most abundant element in air after oxygen and nitrogen. Raindrops contain more dissolved argon than air. Argon is obtained commercially by liquefaction and subsequent fractional distillation of air to separate it from other gases. Argon is two and a half times as soluble in water as nitrogen, and about the same solubility as oxygen. Argon is characterised by by its red spectral lines. Argon is used in electric light incandescent filament bulbs as an inert filling, and in radiation counters, fluorescent tubes, and in argon lasers where, singly-ionized, it produces a characteristic strong emission at 488nm, 514.5nm and 496.5nm at the green-blue end of the spectrum. It is used in electric arc argon welding, where, being inert, it provides a protective gas shield to prevent oxidation of the metals being welded. Also used as a protective blanket for the production of titanium and other reactive elements, and as a protective atmosphere for growing silicon and germanium crystals. Argon can form clathrate 'compounds' where the argon is trapped inside a cage of other molecules, as in Ar8(H2O)6 and Ar(quinol)2, where Van der Waals forces hold on to the argon atoms. Ion molecules such as (ArKr)+, (ArXe)+, (ArNe)+ have been observed spectroscopically. The first covalently bonded compound of argon, hydrogen argon fluoride, HArF, was made in 2000 by cooling argon to just 10 Kelvin, mixing it with hydrogen fluoride, and firing lasers at the mixture whilst warming it slightly. Naturally occurring argon is a mixture of three stable isotopes, 99.6% of which is argon-40, the rest being argon-36 and argon-38. Claim to fame:
|
![]()