
|
17 CHLORINE Cl (Greek: chloros = pale green)
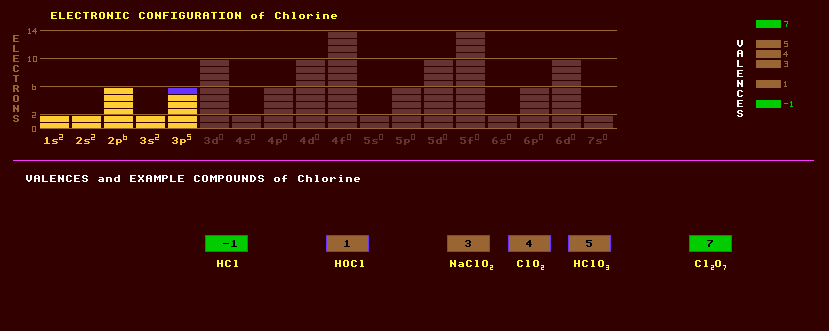
Chlorine is yellowish green poisonous gas with an irritating smell, used as a poison gas in WW I. It is one of the halogens and is a powerful oxidizing agent widely used as a bleach and disinfectant. Chlorine exhibits a multiplicity of valences, 1,3,4,5,6,7 and forms many varied compounds. Known oxides: Cl2O, chlorine monoxide, a brown yellow gas similar to chlorine, which dissolves in water forming hypochlorous acid, HClO. ClO2 chlorine dioxide, an orange-yellow explosive gas similar to chlorine which also bleaches. Cl2O6, chlorine hexoxide, a dark red explosive liquid formed by exposing chlorine and ozone to light. Cl2O7, chlorine heptoxide, a colourless liquid which forms perchloric acid, HClO4, with water, is more stable that Cl2O or ClO2. Known acids: When illuminated, chlorine gas reacts explosively with hydrogen, creating the gas hydrogen chloride, HCl, a strong acid, producing chloride salts, like sodium chloride, NaCl. Hypochlorous acid, HClO, is a weak acid giving rise to salts known as hypochlorites, used as household bleach. Chlorous acid, HClO2. Chloric acid, HClO3, makes chlorates like ammonium chlorate, NH4ClO3. Sodium chlorate NaClO3, used as a weedkiller, is deliquescent and explosively unstable. Perchloric acid HClO4, makes perchlorates like sodium and potassium perchlorates, which are more stable that chlorates, and are used as oxidizers in fireworks.
As perchloroethylene, C2Cl4, contained in huge tanks in underground mines to shield it from muons, chlorine is used to detect solar neutrinos (37Cl + During World War I, phosgene, carbonyl chloride, COCl2, was used as a poison gas. Chlorine can make a great variety of things like plastics; polyvinylchloride, PVC; chloroform or trichloromethane, CHCl3, an anaesthetic; and freons and refrigerants. Upper atmospheric chlorine monoxide (mainly from freon propellants in aerosol cans) is catalysing the destruction of the Earths' protective ozone layer by photochemical reactions. When chlorine compounds are burnt below 900 Celsius, they invariable make dioxins, which are notoriously persistent and the most powerful poisons known to man, which contaminate various lands especially near incinerator plants which sometimes burn at below regulation temperatures. Chlorine is present mainly as sodium chloride, NaCl, common salt in the ocean. Chlorine gas is obtained by the electrolysis of a saturated solution of salt, or brine. It does not occur naturally in elemental form. Chlorine exists as a mixture of two stable isotopes, 75% Chlorine-35, 25% Chlorine-37. A trace amount of chlorine-36, halflife 0.3 Myr, is produced by cosmic ray bombardment. Claim to fame: Chlorine has the highest oceanic abundance (excepting hydrogen and oxygen), the longest oceanic residence time (400 million years), and the highest electron affinity (349KJ/mol).
|
![]()