
|
16 SULPHUR S (Latin: sulphurium)
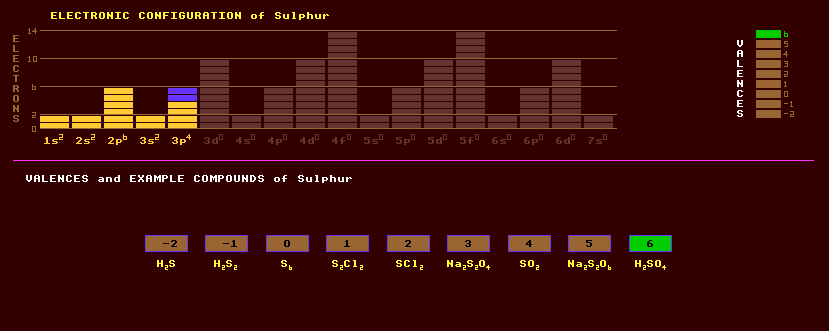
Sulphur is a non-metallic solid that can exist in many allotropic forms. Rhombic sulphur (alpha) is a lemon-yellow powder with a melting point of 113 Celsius and a density of 2.07, monoclinic (Beta) sulphur has a deeper colour and slightly higher melting point and slightly lower density. When melted, sulphur changes to a deep-red rubbery mass called plastic sulphur or gamma sulphur, which has a helical structure with eight atoms per turn; it very slowly reverts back to Rhombic sulphur when cool which consists of eight membered rings, S8. Many allotropes consist of these same eight-membered rings arranged in different crystallographic configurations. The other allotropes of sulphur are complex and number over 20, and most are poorly researched. Sulphur is a very bad conductor of heat or electricity. As flowers of sulphur (powdered sulphur) it is used in gunpowder and fireworks. Sulphur is used to vulcanize rubber (introduce cross links) making it hard and resistant to chemicals. The red coloured polycyclic compound with phosphorus, P4S3, is used in strike-anywhere match heads, with potassium chlorate, KClO3 as oxidant. Sulphur burns with a blue flame emitting poisonous fumes of acrid smelling sulphur dioxide, SO2, which will dissolve in water to produce sulphurous acid, H2SO3, a weak acid whose salts are called sulphites. Sulphur dioxide is used as a sterilizing gas in home brewing (where it is obtained by mixing sodium metabisulphite with citric acid), and as a preservative in many dried foods. Sulphur trioxide, SO3, dissolved in water yields the much stronger sulphuric acid H2SO4, from which sulphates are derived. A green sesquioxide is known, S2O3 as are the acids: sulphoxilic, H2SO2; hyposulphurous, H2S2O4; sulphurous, H2SO3; disulphurous, H2S2O5; thiosulphuric, H2S2O3; permonosulphuric, H2SO5; perdisulphuric, H2S2O8; and thionic acids, H2SxO6; where x is 2, 3, 4, 5, or 6. Sulphur can occur in native form as large pyramidal or tubular transparent yellow crystals which are so fragile they often crack by the warmth of the hand, but usually as sulphides and sulphates around the gaseous vents of volcanoes and hot springs. Most metal sulphides are metallic looking such as iron pyrites FeS2, galena PbS, cobaltite CoAsS, stibnite Sb2S3, chalcopyrite CuFeS2, tetrahedrite Cu12Sb4S13, tennantite (Cu,Fe)12As4S12, chalcosine Cu2S, arsenopyrite FeAsS, bournontite PbCuSbS3, or molybdenite MoS2 whereas others are brightly coloured such as cinnabar HgS (red), greenockite CdS (greenish), orpiment As2S3 (yellow), realgar As4S4 (bright red), and proustite Ag3AsS3 (rich scarlet). Some sulphate deposits, for example copper sulphate, Cu2SO4, were oxidised from the sulphides by bacterial action. Sulphur, under the extreme pressure of 93 GPa or about 10,000 times atmospheric pressure, can un-expectedly turn superconductive, with a critical temperature of 10.1 Kelvin, and at the even higher pressures of 162 GPa the critical temperature rises to an elemental record of 17 Kelvin. Sulphur is obtained either naturally from around volcanic vents or by heating the ore iron sulphide (iron pyrites or fools gold, which resembles gold). Major new sources are extraction from natural gas and from the crude oil used for the manufacture of petrol. It is used in the manufacture of sulphuric acid, H2SO4, and carbon disulphide, CS2. Chemically, sulphur resembles oxygen and can replace it in many compounds. Sulphur can exhibit the valences of 2, 4 or 6. Four hydrides of sulphur are known. Hydrogen sulphide, H2S, an extremely poisonous gas which smells of rotten eggs can be made by adding iron sulphide FeS2 to a weak acid (stink bombs). It is an insidiously dangerous poison in that it quickly deadens the sense of smell before causing death by respiratory paralysis. Other hydrides are H2S2, H2S3, H2S4, and H2S5, etc. With fluorine, it forms sulphur hexafluoride, SF6, an inert gas used for electrical isolation in circuit breakers. Dimethylsulphoxide or DMSO, (CH3)2SO, once hailed as a wonder external painkiller but then found to be poisonous, is produced naturally by organisms in the sea, and is the main source of sulphate in the air. Sulphur can form foul smelling mercaptans, M.SH, as in ethyl mercaptan, C3H5SH, a liquid with a nauseous odour even in minute concentrations. Ammonium sulphate, (NH3)2SO4, is used as a fertiliser. An aqueous solution of copper sulphate, Cu2SO4, will copper plate steel. Alkyl sulphonates are used as detergents in washing powders. Boiling sulphur, at temperature of 444.6 Celsius, is used as a calibration temperature in platinum resistance thermometry. Molten sulphur and molten sodium are used in high-temperature sodium sulphur secondary batteries, which have greater energy density than lead-acid batteries, but need heating before they will work and are much more dangerous. Pyrrotite, marcasite and pyrite are polymorphs of FeS2 crystallizing in the hexagonal, orthorhombic and cubic systems respectively, all are sources of sulphur. The eight-membered ring compound, tetrasulphur tetranitride, S4N4, is explosive when struck or heated and which can be polymerised to (SN)x which exhibits one-dimensional electrical conductivity and unusual optical properties and becomes superconducting at 0.26 Kelvin. A remarkable property of Hydrogen Sulphide on mice (and perhaps other inverterbrates including people) is that when they are exposed to the gas at a concentration of just 80 ppm, they enter a state resembling hibernation, where the metabolic rate plummets to 10%, heart rate slows to 1/75th of normal and body temperature drops to just 15C rather than 37C; a state of suspended animation. Moreover, unlike hibernation, which takes about an hour, the hydrogen sulphide accomplishes in just 3 minutes. If it works in humans, this could help save lives, but it is known that hydrogen sulphide is extremely toxic killing sewage and petrochemical workers accidentally exposed to the gas, and the observed slowing of metabolic rate in mice could merely be a response to poison. Sulphur exists as four stable isotopes, 95% of which is S-32, the rest being S-33, -34 and -36. Eight radioactive isotopes are known, from S-29 to S-40.
Claim to fame: Sulphur has the highest electrical resistivity of any element (2x1023
|
![]()