
|
15 PHOSPHORUS P (Greek: phosphoros = bringer of light)
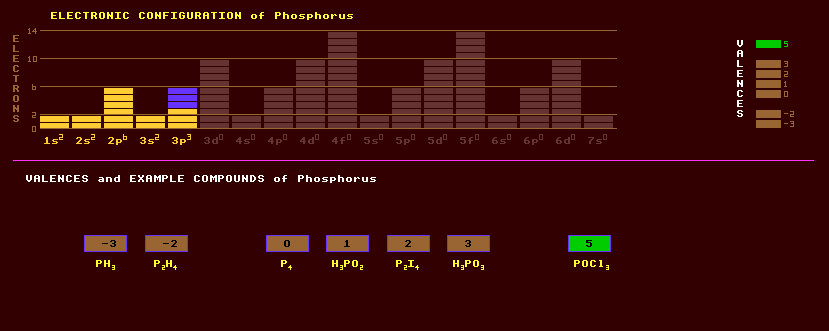
Phosphorus is a non metallic element which can exist in four different allotropic forms, some of which occur free in nature. White phosphorus has a melting point of 44 Celsius is is based on the tetragonally shaped P4 unit, and is a waxy, poisonous solid which ignites spontaneously in air forming phosphorus pentoxide. Red phosphorus is non-poisonous and ignites in air only when heated above 300 Celsius. Violet phosphorus has a higher density and melting point and black phosphorus is a black metallic substance obtainable only at high temperatures and pressures. Elemental red phosphorus was used in matches until it was discovered it caused a medical condition known as 'phossy jaw', but is now chemically combined with sulphur, to form the red coloured polycyclic compound, P4S3, which, with potassium chlorate KClO3 as oxidant, is used in strike-anywhere match heads. Other polycyclic sulphides are P4S4, P4S5, P4S6, P4S7 and P4S9. Phosphorus exhibits valences of three or five, and can form many varied compounds. So called phosphorus pentoxide, but in reality the adamantane-shaped molecule P4O10, is a white poisonous substance and is used as a fertilizer. It is hygroscopic with a great affinity for water, with which it forms phosphoric acid, H3PO4, and is thus used as a desiccant. Some steel is dipped into phosphoric acid to inhibit corrosion. There are salts of three orthophosphates, MH2PO4 which are acidic, M2HPO4 which are neutral, and M3PO4 which are alkaline, in aqueous solutions. Phosphorus trioxide, P4O6, mixed with cold water forms phosphorus acid, H3PO3, whose salts are called phosphites. Other acids are: hypophosphorous, H3PO2; hypophosphoric, H4P2O6; pyrophosphoric, H4P2O7; metaphosphoric, HPO3; permonophosphoric, H3PO5; and perdiphosphoric, and H4P2O8. Some compounds of phosphorus are phosphorescent, that is they absorb light and re-emit it later as a greenish glow. Phosphorus is commonly found as phosphates (especially calcium phosphate from bird droppings) in dry areas of the world, and is also present in plants and animals (where it occurs in bones as apatite, Ca5(Cl,F)(PO4)3, mineralised calcium phosphate). Phosphorus is obtained from calcium phosphate by heating it in an electric arc furnace to about 1450 Celsius with sand and carbon. Phosphates are deliberately added to drinking water supplies to prevent the leaching of metal atoms from household water pipes. The phosphates form a surface coating of insoluble complexes with the copper, lead or iron of the pipes, preventing leaching of poisonous metals from the pipes. Phosphorus is added to bronze making phosphor bronze which is cast to produce slightly porous bearings capable of holding lubricating oil. The hydride, phosphine, PH3, otherwise known as Will o'th Wisp which rises from marshes and stinks, ignites spontaneously in air. Phosphorus dihydride, P2H4, the analogue of hydrazine, is a clear liquid. Phosphorus can be combined with gallium and arsenic to produce red emitting LEDs in GaAsP devices, or used as a dopant of silicon to make n-type semiconductor. A range of organophosphate compounds are used as sheep dip, insecticide sprays and nerve gases, but are extremely poisonous to man (and probably beast). Phosphorus exists as just one stable isotope, phosphorus-31. In addition, 11 radioactive isotopes are known, from P-26 to P-38. Claim to fame:
|
![]()