
|
14 SILICON Si (Latin: silicis = flint)
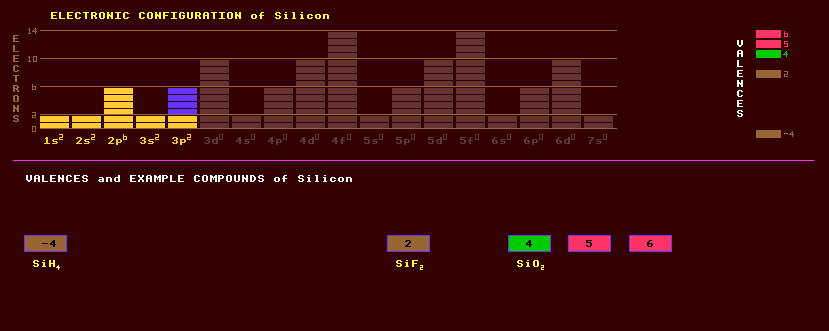
Silicon does not occur naturally in elemental form, but is the second most abundant element on Earth, being present in rocks as metallic silicates, and in sand as silicon dioxide, SiO2, silica. Silicon is obtained by the reduction of silica by carbon in an electric carbon arc furnace at 2200 Celsius. Crystalline silicon is a grey semiconducting non-metal with a valency of four, crystallizing in the cubic system isostructural with diamond. Amorphous silicon is a brown powder. Alloyed or doped with arsenic or phosphorus it produces an n-type semiconductor, doped with boron, aluminium, gallium or indium it produces a p-type semiconductor. Junctions of these semiconductors are used within rectifiers, transistors, integrated circuits, diodes, light emitting diodes and solar cells. FETs use an insulating layer of silicon dioxide to obtain an extremely high input resistance. Silicon changes from being a semiconductor when solid to a metal on melting, which is strange behaviour. Thin alternate layers of silicon dioxide (low refractive index) and titanium dioxide (high refractive index) are used to coat photographic and spectacle lenses to both reduce light reflection from air-glass interfaces, and to increase optical transmission. Previously, magnesium fluoride or cryolite (magnesium aluminium fluoride) were used, but they are too soft. Being tetravalent like carbon, silicon produces analogous compounds to carbon compounds. A whole range of inflammable hydrides can be formed like silane, SiH4. Silane is used to purify silicon to the extremely high purity levels required in silicon foundry IC production. No other element is available in such high purity as silicon. Silicon carbide, SiC, has found use as a semiconductor and the large band gap energy can produce LEDs emitting the more energetic blue-light. The very high melting point of silicon carbide ensures its use in the future as the semiconductor for specialist integrated circuits wherever high temperatures are likely to be encountered, eg satellites, rocket engines and car engines. Silicon carbide is also extremely hard and so is used as a very hard abrasive. Silicon dioxide, silica, is widely distributed and occurs in many forms and minerals: quartz (crystallizing in the hexagonal trigonal system) is a clear crystal occurring with many ores but especially associated with galena, lead sulphide. Amethyst (violet), citrine (yellow to brownish caused by ferric oxide impurities), smoky quartz (brownish to blackish caused by radioactively induced dislocations of silicon atoms), and rose quartz (pinkish due to manganese oxide) are different colours of quartz; chalcedony, heliotrope, hornstone, jasper, onyx, and agate are different varieties often variegated with intricately coloured patterns. Chalcedony is variously coloured: yellow red (carnelian and sard), green (chrysoprase and plasma) but commonly grey. Opal is an amorphous hydrous silicon dioxide, SiO2.nH2O. Amethyst owes its purple to red/violet colour to iron and radioactive irradiation. The colour of amethyst can fade, heating it will change its colour through yellow to brown, leak-green (prasiolite) and finally colourless tones. Golden topaz is a misleading or fraudulent name given to amethyst or smoky quartz which has been turned yellow by heating to 250 Celsius. Under more extreme conditions of temperature and pressure, the much rarer tridymite (orthorhombic) and cristobalite (tetragonal) are formed, which are polymorphs of quartz. Two other polymorphs of silica found on Earth, coesite and stishovite, are formed under extremely high pressure by meteoritic impact. Unlike quartz, the silicon within stishovite is 6-coordinated into an octahedral shape rather that 4-coordinated into a tetrahedral shape. Silica is used in the manufacture of glass and borosilicate glass. Sandy beaches are composed almost entirely of pure quartz, the other softer minerals from the pulverised rocks having long since dispersed. Quartz crystals are piezoelectric, where a mechanical strain generates an electrical charge, and were used for gramophone pickups and microphones, but more recently for accelerometers, accurate crystal oscillators for computers or watches, and electronic filters. Quartz is also optically active and able to rotate the plane of polarization of light, useful in polarisers. Quartz is transparent to ultraviolet light and is used as windows in EPROMs and sun-ray lamps. Quartz beakers are used to contain hydrofluoric acid because they are unaffected by it. The hollow needles of stinging nettles are made of quartz. Zeolites are porous rocks which are being increasingly used for highly selective absorption of pollutants or other, particularly ionic, substances and for highly specific catalysis. Zeolites are hydrous aluminosilicates (where aluminium substitutes for some atoms of silicon) in which the water can be driven off by heating leaving the crystal structure undisturbed and with precisely shaped hollow sites which are capable of very selective catalysis of certain reactions or of absorbing specific ions. Not all aluminosilicates are zeolites. Zeolite ZSM-5 is used commercially to convert methanol to petrol, but exactly how it performs this complex task is unknown. Silicates form a diverse ubiquitous group consisting of perhaps half of all minerals. Silicates are sub-divided into the following types: silica SiO2 with a 3-D framework structure; disilicates Si2O5 with a sheet structure; four linear-chain metasilicates Si3O8, Si4O11 and SiO3 (with triple, double and single chains respectively), the fourth (SiO3)n having a ring structure; pyrosilicates Si2O7 which are in isolated groups of tetrahedrons; orthosilicates SiO4 in isolated single tetrahedrons; and subsilicates SiO5 in isolated tetrahedrons with additional oxygen atoms. Major silicates of the silica type are the feldspars which are aluminium silicates containing potassium, sodium or calcium; and the zeolites described above. The disilicate types include kaolin, chrysotile asbestos and micas. The metasilicates include amphiboles, pyroxenes, pyroxenoids, actinolite asbestos, the tourmaline group and beryl. The pyrosilicates include hemimorphite and danburite. The orthosilicates include the olivine series, humites, epidotes, garnets and also the minerals zircon and topaz. The subsilicate group includes titanite, and uranophane. Silicon is a mixture of three stable isotopes, silicon-26, which is by far the most abundant, and silicon-27 and Si-28. In addition, 10 radioactive isotopes are known, from the positron emitting Si-24 to the electron emitting Si-36. Claim to fame: Because of its use in electronics, silicon is available in a higher purity grade than any other element.
|
![]()