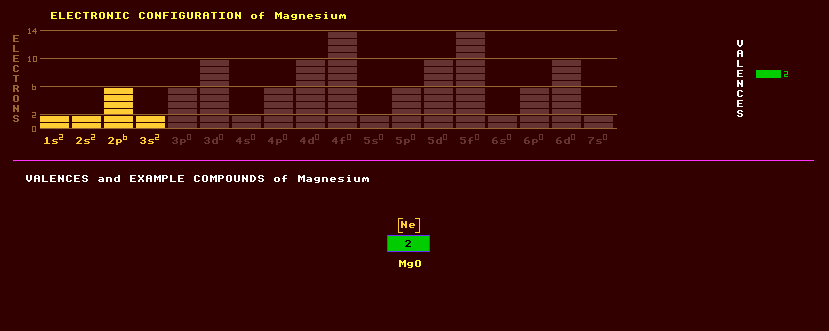

|
12 MAGNESIUM Mg (Greek: magnesia, district of Thessaly)
Magnesium is a lightweight, shiny white, malleable, ductile alkali metal, which when ignited, burns fiercely in air producing a brilliant white light and clouds of white fumes of magnesium oxide, MgO. Milk of magnesia is a liquid suspension of white magnesium oxide powder taken medicinally for relief from indigestion. Magnesium oxide is a very refractory material able to withstand intense heat, and is used to line furnaces. Powdered magnesium was once used for illumination in open flash photography, and is still used in magnesium distress flares and for pyrotechnics at rock concerts. Magnesium is a very light metal, with a specific density of only 1.74, a third lighter than aluminium with which it is alloyed to increase the strength of the duralumin so formed, used in aircraft frames. Magnesium is used as a sacrificial anode for preventing corrosion of metal-hulled craft by salty sea water and also as a reducing agent in the purification of uranium and other metals from their salts. Magnesium diboride, MgB2 has the highest superconducting transition temperature of any metallic alloy known as of 2006, being 40 Kelvin. Superconductivity in metals is caused by the movement of electrons through the material setting up vibrational energy in the atomic lattice, which causes a shift in charge such as to attract a second electron to the first. The two electrons are then paired up. It is this pairing of electrons which allows the electron pair to move through the atomic lattice without bumping into any atoms in the lattice, which would then cause electrical resistance. The vibrations in the atomic lattice are phonons. Despite this record breaking superconducting temperature theoreticians calculate that in magnesium dibromide only 3% of the electrons are paired up, and if they can find a material with better matching between phonon and electron-pair vibration frequencies, the coupling electron pairing could reach 100% where it should be possible to achieve a suerconducting temperature as high as an amazing 430 Kelvin. It is just a metter of finding a material that has 100% coupling efficiency. There is no guarantee that such a material exists, after all there are only 92 odd elements from which to make any such alloy.
Magnesium forms salts with acids, which crystallize mainly as white solids. Magnesium sulphate, MgSO4·7H2O, commonly known as Epsom salts, is used medicinally. Magnesium carbide, Mg2C3, liberates allylene gas, CH3-C Magnesium can be extracted from carnallite, a hydrated potassium magnesium chloride, KMgCl2·6H2O; from brucite, magnesium hydroxide, Mg(OH)2; from magnesite or bitter spar, magnesium carbonate, MgCO3, from which heat resistant bricks are made for the refractory lining of blast furnaces; from dolomite, calcium magnesium carbonate, CaMg(CO3)2, the marble found in the Italian Dolomites, but is now extracted electrolytically from sea water. Like aluminium, magnesium is a very electropositive metal and is strongly lithophilic, or rock forming. Magnesium occurs in the pink semi-precious gem stone mineral, ruby spinel, a magnesium aluminate, MgAl2O4; in the dark red gemstone pyrope, a magnesium aluminium silicate, Mg3Al2(SiO4)3; in talc, Mg6[Si-O20](OH)4, a soft and hydrous magnesium silicate used in talcum powder; in forsterite, Mg2SiO4, a magnesium silicate belonging to the olivine series, (Mg,Fe)2SiO4; in enstatite, MgSiO3, a magnesium silicate belonging to the pyroxene series, (Mg,Fe)SiO3; in epsomite, magnesium sulphate, MgSO4.7H2O; in cordierite, magnesium aluminium silicate, Mg2Al4Si5O18 which is pleochroic, appearing blue or violet from certain angles; and many others including diopside, tourmaline, nephrite, asbestos, mica, and vermiculite. Serpentine or chrysotile, a complex basic magnesium silicate, Mg6(OH)8Si4O10, is a microscopically fibrous mineral consisting of chrysotile, the common form of asbestos. Asbestos, which is really a term for three or four different fibrous minerals, can withstand great heat used for fireproofing. Vermiculite, a hydrated magnesium iron aluminium silicate, (Mg,Fe,Al)3(Al,Si)4O10·4H2O, is a hydrated mica type mineral consisting of golden yellow plates which, on heating to 300 Celsius, lose water and swell enormously such that it floats on water, in which form it is used as thermal insulation, and also in 'gold' paints and plastics. The weathering of magnesium containing rocks results in a substantial magnesium ion (Mg2+) concentration in fresh water comparable to that of sodium and potassium. The residence time for oceanic magnesium is a very long 10 million years. Magnesium exists as a mixture of three stable isotopes, magnesium-24 magnesium-25 and magnesium-26 in a 8:1:1 ratio, respectively. In addition, 12 radioactive isotopes are known, ranging from Mg-20 to Mg-34. Claim to fame:
|
![]()