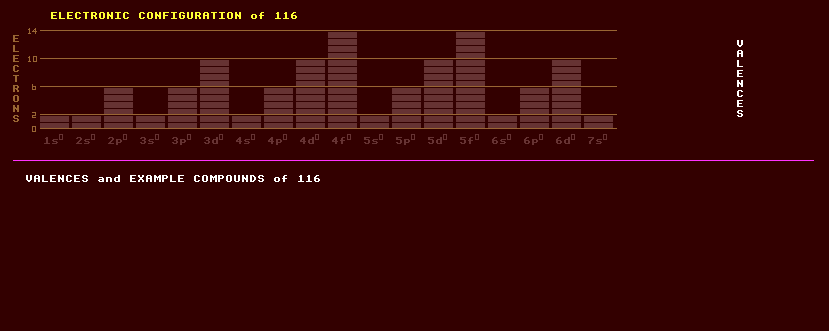

|
116
Three atoms of element 116 were produced and detected in June 1999 by a team working at The Lawrence Berkely Laboratory in America as a by-product in the alpha decay of element 118 which they had just made. By crashing a beam of krypton-86 atoms into a target of lead-208 atoms several excited compound nuclei were produced. These almost immediately emit a neutron becoming atoms of element 118 with 118 protons and 175 neutrons, being atoms of the isotope 118-293. Less than a millisecond later the isotope 119-293 decays by alpha decay into an atom of element 116, the isotope 116-289. This isotope also decayed by alpha decay into element 114, the isotope 114-285. Along with oxygen, sulphur, selenium and tellurium, element 116 belongs to group 16. new: 116UUH292 resulting from the reaction of 96Cm248Cm with 20Ca48: 96Cm248 + 20Ca48 → 116Uuh292 + 40n1
This isotope of Element 116, 116Uuh292, decayed in 47 milliseconds to a previously identified isotope of Element 114, 114Uuq288: 116Uuh292 → 114Uuq288 + 2He4 Claim to fame:
|
![]()