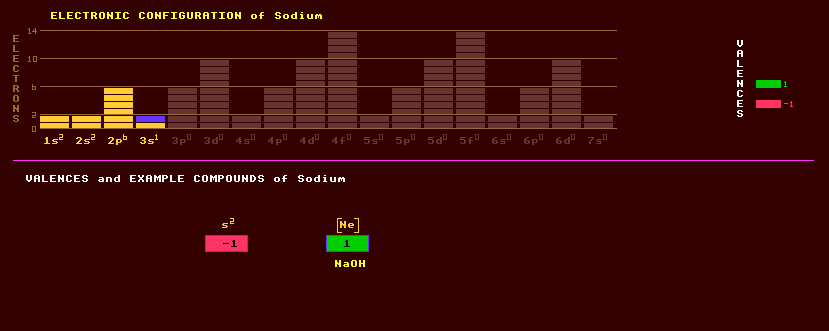

|
11 SODIUM Na (Latin: Natrium)
Sodium is a soft, bright, silvery, highly reactive alkali metal which tarnishes quickly if exposed to air and reacts violently with water, producing hydrogen gas and sodium hydroxide, NaOH, a powerful alkali and bleach. Sodium burns in air with a brilliant white flame. It is just lighter than water and can be cut with a knife. Sodium is used in some nuclear power stations as the coolant, because when molten, at a temperature above 98 Celsius, it can transport more heat than water, but it can explode if water is allowed to get in contact with it, as happened in decommissioning a French nuclear reactor in 1994. When alloyed with potassium to form NaK, its melting point decreases below that of either element alone such that it is molten at room temperature, and is used as a coolant. Sodium is kept under oil. It is also proposed for use as the electrochemical fuel in sodium sulphur batteries, which can store more electrical energy than can lead acid accumulators, but again only works when the sodium is in its molten state. Sodium is used in street lamps where its intense yellow D-line emission is obvious. Sodium forms ionic-bonded crystals with the halogens, for instance, sodium chloride, NaCl, also known as common salt which is essential for human life. Sodium, along with potassium, is used by nerve cells in the conduction of nerve impulses. Sodium deficiency causes muscular spasms or cramp. Excess salt contributes to high blood pressure and can be lethal, for instance, when only sea water can be drunk. Salt crystallizes in the cubic system and is famous for the stepped hopper shape of its crystal form. Salt is found dissolved in the seawater and in huge underground evaporite deposits in Cheshire, which was once covered by shallow sea waters when England was on the equator. Sodium is the most abundant metal ion dissolved in sea water. Evaporite deposits of sodium carbonate, sodium sulphate and sodium borate are also known. Sodium forms many other salts with acids. Sodium silicate, water glass, was once used for preserving eggs. Sodium chlorate, NaClO3, is deliquescent and used as a weed killer and as an oxidant, but is rather unstable when dry and can explode, as happened in Salford when it was stored in large oil drums. Sodium perchlorate, NaClO4, is more stable than the chlorate, and is used as the oxidant in some fireworks. Sodium nitrate, NaNO3, is called Chile saltpetre. Sodium hydroxide, NaOH, or caustic soda is used extensively. Sodium thiosulphate, hypo, Na2S2O3·5H2O is used in photographic development. Borax, Na2B4O5·10H2O, is used as a flux in welding, an ant poison, and medicinally. Sodium forms many other important compounds like sodium sulphate, NaSO4, sodium carbonate, Na2CO3 or soda ash; sodium bicarbonate or baking soda, NaHCO3, is used to raise cakes. Rochelle salt is sodium potassium tartrate, and exhibits piezoelectric properties and was used as a bimorph in early gramophone pickup cartridges, microphones and tweeters, but it has a low Curie point of only 23 Celsius, above which it temporarily loses these properties, and has now been superseded by barium titanate. Sodium cyanide, NaCN; sodium peroxide, NaO2, sodamide, NaNH2; and sodium hydride, NaH are also important compounds. Sodium is used in the manufacture of glass, (sodium silicate), and (sodium) borosilicate glass. Sodium is a powerful reducing agent used in the extraction of titanium and zirconium, and in the synthesis of tetraethyl lead. Many silicate minerals contain sodium, including plagioclase feldspars, (Na,Cl)(Al,Si)4O8. Because most compounds of sodium are soluble in water, it is easily weathered out finding its way into sea-water. Sodium has in the past been extracted from soda nitre; cryolite, sodium aluminium fluoride, Na3AlF6; amphibole; zeolite; and sodalite, sodium aluminium silicate chloride, Na4Al3(SiO4)3Cl, but is now obtained by the electrolysis of dry fused sodium chloride, itself got from the sea. This process is much cheaper than electrolysing sodium hydroxide as was done previously. Sodium comprises just one stable isotope, sodium-23. In addition, 11 radioactive isotopes are known, ranging from Na-20 to Na-35. Claim to fame:
|
![]()