
|
105 DUBNIUM Db (Dubna, Moscow) [Hahnium, Ha - Otto Hahn, German physicist]
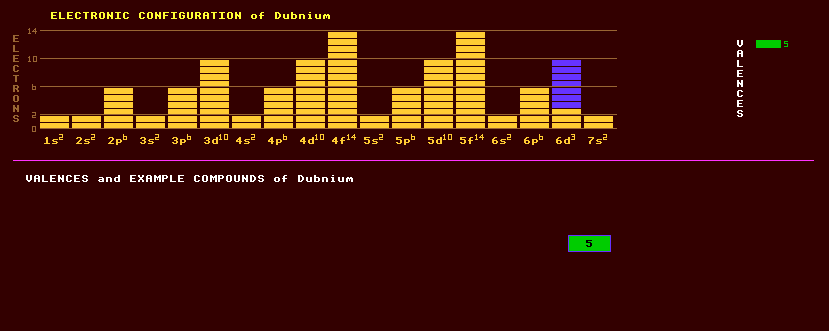
An artificially produced, highly unstable, transuranic element with no detectable natural occurrence on Earth. Element 105 or dubnium, as it is now called, was first identified in 1967 by a group of researchers at the Joint Institute for Nuclear Research in Dubna, Russia, by bombarding americium-243 with neon-22 ions producing dubnium-260, which decays by either spontaneous fission or alpha decay with a halflife of 1.5 seconds, and dubnium-261 which again decays by either SF or alpha decay with a halflife if 1.8 seconds. Alpha-alpha correlation, in which the alpha decay of the unknown isotope and that of its alpha decay products are correlated in time, can be used to positively identify short lived isotopes and was used by Ghiorso at the Berkeley Heavy Ion Linear Accelerator in America to identify dubnium. They bombarded a target of californium-259 with a beam of nitrogen-15 ions at an energy of 84 MeV fusing the two nuclei with the emission of four neutrons to produce dubnium-260. Dubnium-261 was produced in 1971 by bombarding either a californium-250 target with nitrogen-15 ions, or by bombarding a berkelium-249 target with oxygen-16 ions. It was only named dubnium in 1993. Chemical studies of dubnium, one atom at a time, are underway using the longest lived known isotope, dubnium-262. Dubnium belongs to group 5 with vanadium, niobium, and tantalum with which it shares many chemical similarities. However, it behaves less like its closest analogue tantalum and more like niobium its more distant relative and protactinium, an actinide with pseudo-group 5 properties. Dubnium sorbs on glass like other group 5 homologs like niobium or tantalum, but the complexing properties of dubnium differ from those of tantalum. It seems that, like other group 5 elements, its most stable aqueous valency is +5. The reason that very heavy elements have slightly differing chemical properties to their closest members of the same group is because of the very high electrical charge of their nuclei, which attracts the inner electrons closer to the nucleus, where they acquire enormous relativistic velocities and hence gain extra mass. Thus the s- and p-shell electrons are attracted closer to the nucleus where they better shield the d- and f-shell electrons from the nuclear charge, which then move further out, altering the chemical properties of the heaviest elements. The isotope of dubnium with the longest known halflife is dubnium-262, which is produced by bombarding a thin berkelium-249 target with oxygen-18 ions in the Berkeley 88 inch cyclotron. On average, 3×1014 projectiles of oxygen-16 on target with berkelium-249 produce by fusion 3×107 compound nuclei of dubnium-267, which evaporates five neutrons to produce just one atom of dubnium-262. The halflife of dubnium-262 is just 34 seconds, it decays by either alpha decay into the alpha decaying lawrencium-258 which has a halflife of 4 seconds or by electron capture to rutherfordium-262 which decays by spontaneous fission with a halflife of just 4.7 milliseconds. Altogether, 8 isotopes of dubnium are known, all radioactive, and ranging from dubnium-255 which decays by spontaneous fission with a halflife of 1.5 seconds to dubnium-263 which decays by either spontaneous fission or by alpha decay with a halflife of 27 seconds. One isotope of Dubnium, Db-268, has the very long half-life of 16 hours. Claim to fame:
|
![]()