
|
104 RUTHERFORDIUM Rf (Ernest Rutherford, New Zealand physicist)
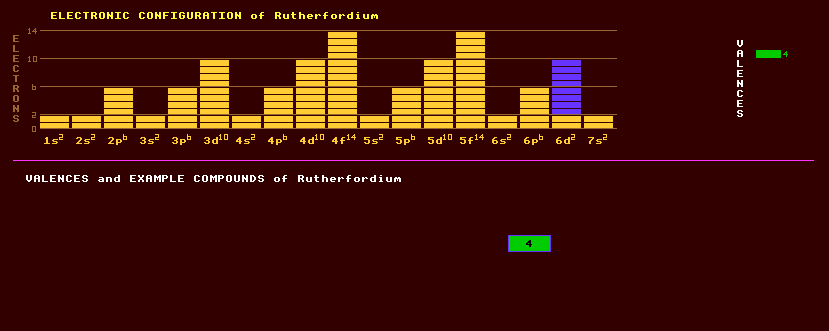
An artificially produced, highly unstable, transuranic element with no detectable natural occurrence on Earth. Once called Kurchatovium, Kv, it was only named rutherfordium in 1993. The first claimed discovery of rutherfordium was of rutherfordium-260, which decays by spontaneous fission with a halflife of 20 milliseconds, by Flerov at Dubna, near Moscow, where he bombarded plutonium-242 targets with neon-22 ions but was reasoning was based on rather dubious inferences. Alpha-alpha correlation, in which the alpha decay of the unknown isotope and that of its alpha decay products are correlated in time, can be used to positively identify short lived isotopes and was used by Ghiorso at the Berkeley Heavy Ion Linear Accelerator in America to identify rutherfordium. They bombarded a target of californium-249 with carbon-12 and carbon-13 ions to produce four free neutrons and rutherfordium-257 which decays by spontaneous fission or by alpha decay with a halflife of 4.8 seconds. Since then, rutherfordium-257 and rutherfordium-256 have been produced at the GSI research facility in Darmstadt, Germany by bombarding a lead-208 target with titanium-50 ion projectiles, creating a compound nucleus rutherfordium-258*, which upon losing one or two neutrons becomes the aforementioned isotopes of rutherfordium. Chemical studies of rutherfordium, one atom at a time, are underway using the longest lived known isotope, rutherfordium-261, an SF/alpha decaying isotope with a halflife of 65 seconds, produced by bombarding a thin curium-248 target with oxygen-18 ions in the Berkeley 88 inch cyclotron. By analogy with hafnium, with which it shares the same group number (4), it might be expected to have a valency of 4+ in aqueous solution, but relativistic calculations of the orbits suggest the electronic configuration may be 7s27p1 rather than 6d27s2 which might lead to a stable valency of 2+. The rutherfordium isotope with the longest known halflife is rutherfordium-261 with a relatively long halflife of 1.08 minutes decaying by alpha decay into the alpha decaying nobelium-257 which has a halflife of 25 seconds. Altogether, 10 isotopes of rutherfordium are known, all radioactive, and ranging from the spontaneous fission decaying rutherfordium-253 which has a halflife of 1.5 seconds to rutherfordium-262 which also decays by spontaneous fission with a halflife of just 47 milliseconds. Claim to fame:
|
![]()