
|
103 LAWRENCIUM Lr (Lawrence - scientist)
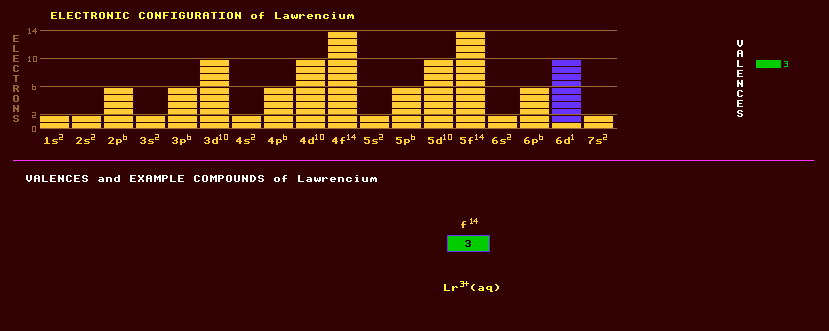
An artificially produced, highly unstable, transuranic rare earth element of the actinide series with no detectable natural occurrence on Earth. Lawrencium was first positively produced and identified by Ghiorso and colleagues at the Berkeley Heavy Ion Linear Accelerator in 1961 by bombarding a thin film of californium isotopes with beams of boron ions. The recoiling products were collected on a moving mylar film which traversed a series of alpha detectors. These detected lawrencium-257 and lawrencium-258 isotopes with halflives of just a few seconds. In 1965 researchers at Dubna used a double recoil technique to identify the longer lived lawrencium-256 isotope, with a half life of 26 seconds. They linked its decay via electron capture and alpha decay processes to its known granddaughter product, fermium-252, with a half life of 25 hours, which was identified chemically and by its characteristic alpha decay signature. Chemical studies of lawrencium, one atom at a time, were underway using the longest lived known isotope, lawrencium-260, with a halflife of 3 minutes, produced by bombarding a berkelium-249 target with oxygen-18 ions in the Berkeley 88 inch cyclotron. Then in 1988 a much longer lived isotope of lawrencium was discovered, lawrencium-262, with a halflife of 3.6 hours, which is now used for chemistry with lawrencium. They confirm that it exhibits a valency of 3+ in aqueous solutions, along with most other actinides, except nobelium with 2+. The longest lived isotope of lawrencium is lawrencium-262 with a halflife of just 3.6 hours decaying by electron capture into nobelium-262 which decays by spontaneous fission with a halflife of just 5 milliseconds. Altogether, 10 isotopes of lawrencium are known, all radioactive, and ranging from the alpha decaying lawrencium-253 which has a halflife of 1.3 seconds to lawrencium-262, mentioned above. Claim to fame:
|
![]()