
|
102 NOBELIUM No (Alfred Nobel, Swedish chemist)
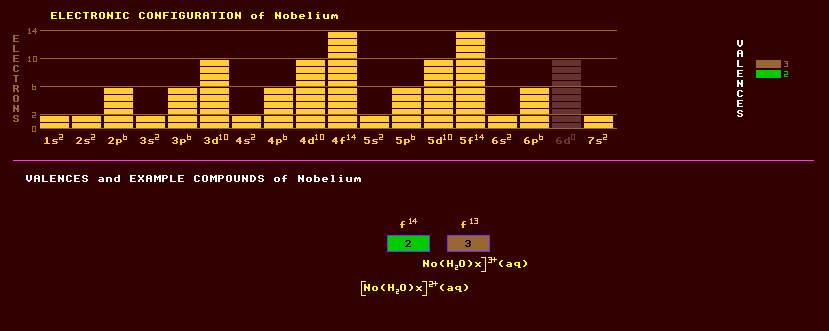
An artificially produced, highly unstable, transuranic rare earth element of the actinide series with no detectable natural occurrence on Earth. The first claim to discovery of Nobelium came in 1957 when an international team from England, Stockholm and America irradiated a film of curium-244 with carbon-13 ions, but their interpretation of the product could have been in error. In 1966 Nobelium was positively identified in the Nuclear research at Dubna, near Moscow, and by another group in America. The isotopes identified were nobelium-254 with a halflife of 55 seconds, nobelium-252 with a halflife of 2.3 seconds and nobelium-257 with a 23 second halflife. Nobelium, once produced an atom at a time, in the form of nobelium-255 can now be produced at a rate several thousand atoms per ten minute period by bombarding a californium-249 target with carbon-12 ions. Nobelium exhibits two valences, +3 and the commonly expressed +2. The only known compound are the complexes which exist only in aqueous solution: [No(H2O)x]2+, and [No(H2O)x]3+. The longest lived isotope of nobelium is nobelium-259 with a short halflife of just 58 minutes decaying by either alpha decay into the alpha decaying fermium-255 which has a halflife of 20 hours or by electron capture into mendelevium-259 which decays by spontaneous fission with a halflife of 1.6 hours. Altogether, 12 isotopes of nobelium are known, all radioactive, and ranging from the spontaneous fission decaying nobelium-250 which has a halflife of just 250 microseconds to nobelium-262 which also decays by spontaneous fission with a halflife of just 5 milliseconds. Claim to fame:
|
![]()