
|
101 MENDELEVIUM Md (Mendeleev, chemist)
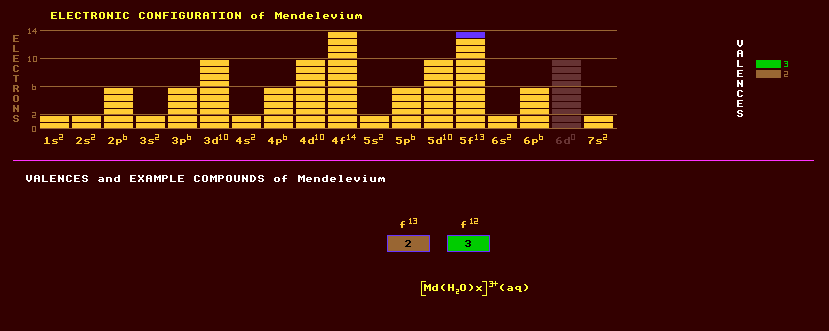
An artificially produced, highly unstable, transuranic rare earth element of the actinide series with no detectable natural occurrence on Earth. First produced in 1955 by Albert Ghiorso at the Lawrence Berkeley Laboratory based on the detection of just 17 atoms. He bombarded a thin film of the highly radioactive and rare isotope of Einsteinium-253 weighing just one picogram (and produced by the neutron irradiation of plutonium) with helium ions to produce Mendelevium-257. He used the recoil method, in which the transmuted isotopes are produced with enough energy to recoil out of the bombarded film, thus considerably reducing the otherwise enormous task of separating a few atoms of the product from the billions of the target and before they have all decayed. This also allows the precious target to be used time and again. The recoiling products were rapidly separated by elution in a column of resin. Trivalent actinides and lanthanides elute from this according to their ionic radius, the smaller ones eluting first. Radioactivity detected at positions corresponding to element 100 and 101 indicated that the nuclei were breaking into two smaller fragments by spontaneous fission. The Mendelevium-257 produced rapidly emitted a neutron becoming mendelevium-256, which then decayed with a half life of about 1.6 hours to become Fermium-256, and which then underwent spontaneous fission with a halflife of about 3 hours. Since then, many millions of atoms of mendelevium-256 have been produced, which have probably all decayed. Mendelevium exhibits two valences, +2 and the commonly expressed +3. A complex [Md(H2O)x]3+ in aqueous solution is known. The longest lived isotope of mendelevium is mendelevium-258 with a halflife of just 51.5 days decaying by either alpha decay into the alpha/beta decaying einsteinium-254 which has a halflife of 276 days or by electron capture into fermium-258 which decays by spontaneous fission with a halflife of just 370 microseconds. Altogether, 14 isotopes of mendelevium are known, all radioactive, and ranging from the alpha decaying mendelevium-247 which has a halflife of just 2.9 seconds to mendelevium-260 which decays by spontaneous fission with a halflife of 27.8 days. Claim to fame:
|
![]()