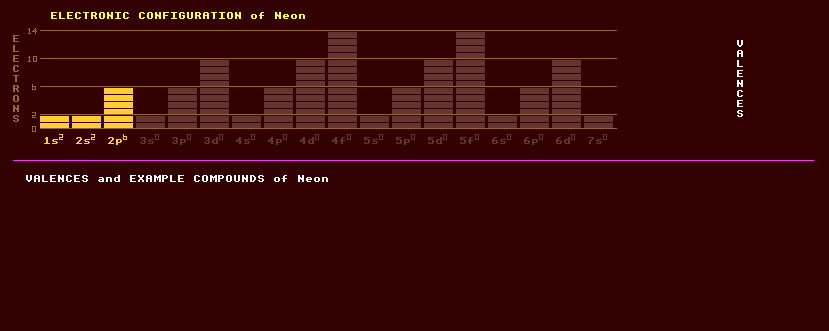

|
10 NEON Ne (Greek: neos = new)
A colourless, odourless noble monatomic gaseous element once thought to be totally inert due to its completed-octet electron shell, but now suspected of forming various compounds under certain conditions. It is present in air at 18 ppm; and is obtained commercially by liquefaction of air and subsequent fractional distillation to separate it from other gases. Of all the rare gases, the electrical discharge in neon is the most vivid and intense at ordinary voltages, its breakdown voltage being just 90 volts. Used in gas discharge lamps under the name of 'neons' where it gives off an orange-red glow useful for signs. Neon is the active lasing gas in helium-neon lasers, which produce coherent red light by electrical excitation. Also used as the gas filling in voltage stabiliser valves because of the existence of a negative resistance region in its voltage versus current characteristics. Once also used as red power indicators on mains driven instruments, but now completely replaced by light emitting diodes. Neon is said to form a compound with fluorine. The following ions are known from spectroscopic studies: Ne2+, (NeAr)+, (NeH)+, and (HeNe)+. Neon forms an unstable hydrate, and can also form clathrate compounds where an atom of neon is mechanically trapped in a cage; one example being a football-shaped sphere of carbon atoms called Buckminster Fullerene, Ne@C60. Liquid neon is finding important application as an economical cryogenic refrigerant, having over 40 times more refrigerating capacity per unit volume than helium, and three times that of liquid hydrogen. It is also less expensive than helium. Neon consists of three stable isotopes, of which Neon-20 is the most abundant, followed by neon-22, with just a fraction of a percent of neon-21. In addition, 8 radioactive isotopes of neon are known, ranging from Ne-16 to Ne-26. Claim to fame: Neon has the lowest cohesive energy (1.92 KJ/mol) of any element (although helium should have if someone measured it).
|
![]()